Facile reduction of aromatic nitro compounds to aromatic amines catalysed by support-free nanoporous silver†
Zhiwen Lia,
Xiaohong Xua,
Xiaojian Jianga,
Yingchun Lib,
Zhixin Yuc and
Xiaomei Zhang*a
aSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China
bSchool of Pharmacy, Shihezi University, Shihezi 832000, China
cDepartment of Petroleum Engineering, University of Stavanger, Stavanger 4036, Norway
First published on 23rd March 2015
Abstract
Nanoporous silver was used as the catalyst for the reduction of aromatic nitro compounds even in the presence of some sensitive functional groups under mild conditions with excellent yields. A reduced amount of NaBH4 was used. The reaction kinetics was studied with the help of UV-visible spectrophotometry.
Nanoporous metals (np-M) made by dealloying have attracted increasing interest due to their broad applications in fuel cell technologies, biomolecular sensing, surface-enhanced Raman scattering (SERS), electrocatalysis, and heterogeneous catalysis. Their unique structural properties of mechanical rigidity, nanoscale three-dimensional bicontinuous porous structure, easy functionalization and high corrosion resistance make these unsupported materials a good choice for green, sustainable catalysts.1–3 To date, some of np-M have been used in the gas phase,4,5 liquid phase6–8 and electrochemical9,10 catalytic reactions and exhibited effective selectivity and catalytic activity. Compared with gas phase and electrochemical catalytic reactions, the investigation of np-M-catalyzed liquid phase catalytic reactions was only developed recently (in 2010). Therefore relative works were rare and most of them limited to nanoporous gold (np-Au). Although other np-M materials were used in a small number of liquid phase catalytic reactions, the results were exciting.11–14 Searching for efficient, highly-selective np-M-catalyzed reaction systems is necessary due to the unique structural properties mentioned above.
Aromatic amines are important substances in synthetic chemistry and chemical engineering.15,16 Reduction of aromatic nitro compounds is one of the main paths to get aromatic amines. Different reductants have been used for this reaction with transition metals as the catalysts. But high temperature and pressure were always required.17–19 Sodium borohydride (NaBH4) was a common reductant for hydrogenation. When it was used for reduction reactions with the help of transition metal catalysts, although normal pressure and room temperature was available, substantial molar excess of NaBH4 relative to the substrates, usually exceeding 100-fold excess, was needed.20–23 It is important to search for a suitable catalyst which could increase the catalytic efficiency and decrease the amount of NaBH4.24
In the present work, we comparatively investigated the catalytic activity of different nanoporous metals for the reduction of aromatic nitro compounds and found that nanoporous silver (np-Ag) exhibiting the highest activity. The aromatic nitro compounds with not only electron-withdrawing groups but also electron-donating groups could be hydrogenated to the corresponding aromatic amines with good yield and high selectivity at room temperature. Compared with other transition metal catalysts, when np-Ag was used as catalyst, only 25-fold excess of NaBH4 was needed. In addition, Np-Ag could be reused at list seven times without the loss of catalytic activity.
The previously reported fabrication method for np-Ag was by etching magnesium from Ag–Mg alloy.25 In our present case, as aluminium can melt with Au, Ag, Cu, Pd, therefore different aluminum-base (Al75M25) alloys were prepared firstly by melting aluminum with other metals (Au, Ag, Cu, Pd). Al75Ag25 was chose as a representative example to be introduced. Sodium hydroxide solution (NaOH, 10 wt%) was used to etch the aluminum from the alloy. After the dealloying process the nanoporous silver was got, as showed in Fig. 1a, nanoscale three-dimensional bicontinuous porous structure was obtained and the average ligament size was around 30 nm. In order to check the chemical state of Ag, XPS of nanoporous Ag before the reaction was detected. As can be seen from the XPS spectrum of Ag 3d region, Fig. 2a, two peaks occurred at 368.55 and 374.55 eV were well corresponded with Ag 3d5/2 and Ag 3d3/2 binding energies, indicating the metallic nature of np-Ag.26
 | ||
| Fig. 1 SEM image of dealloyed np-Ag (a) before catalytic reaction and (b) after being used for seven times. | ||
Nanoporous silver was first used as the catalysts for the reduction of 4-nitrophenol (4-NP) by using NaBH4 as reductant. Ultraviolet and visible (UV/Vis) spectrophotometer was used to analyze the reaction process. As shown in Fig. 3a, the characteristic absorption peak of 4-NP and 4-aminophenol (4-AP) in the presence of NaBH4 was appeared at 400 and 300 nm, respectively. During the reaction, the intensity of the characteristic peak of 4-NP decreased, while the absorption peak at 300 nm attributing to 4-AP increased gradually, indicating the successful reduction of 4-NP to 4-AP. In this reaction, NaBH4 was used in 25-fold excess, so the reaction rate would follow pseudo-first-order kinetics. A linear correlation between ln(At/A0) and reaction time, which At and A0 represented the absorbance at the fixed intervals and the absorbance at the initial stage, respectively, were got, Fig. 3b. This result further confirmed the reaction is a pseudo-first-order and its rate constant (k) was calculated from the slope to be 0.036 min−1.
 | ||
| Fig. 3 Time-dependent UV-visible absorption spectra for the reduction of 4-nitrophenol over nanoporous silver in aqueous media at room temperature, Fig. 2a and plot of ln(At/A0) versus time for the reduction of 4-nitrophenol over nanoporous silver, Fig. 2b. Reactions were performed using np-Ag 0.05 mmol, 4-NP 20 mM and NaBH4 500 mM in 10 mL aqueous solution at room temperature. | ||
Then other nanoporous metal catalysts were used to reduce the 4-NP under the same conditions (Table 1, Entry 2–5). Corresponding rate constant (k) of the catalysts was also obtained, and a plot of ln(At/A0) versus time were shown, (Fig. S1–S4, ESI†). Meanwhile, the turnover frequency (TOF) of each catalyst was calculated, (the calculation was showed in ESI). Compared with np-Ag–Al, the nanoporous silver fabricated from the magnesium–silver alloy (np-Ag–Mg) show lower activity. This result would most originate from the difference in their sacrificial metal (Al vs. Mg), which effort the structure and the surface state of the catalysts. At the same mol percent, the nanoporous palladium (np-Pd) exhibited the similar activity to np-Ag–Al, but the TOF was lower as the specific surface area was large. Due to the rapid decomposition of NaBH4 on the surface of nanoporous gold (np-Au), the activity of np-Au (k = 0.028 min−1) was lower than np-Ag. The nanoporous copper (np-Cu) showed limited activity (k = 0.017 min−1). Np-Ag–Al exhibited highest activity among the nanoporous materials we used.
| Entry | Catalyst | k/min−1 | BET/m2 g−1 | TOF/min−1 |
|---|---|---|---|---|
| a Reactions were performed using catalyst 0.05 mmol, 4-NP 20 mM and NaBH4 500 mM in 10 mL aqueous solution at room temperature. | ||||
| 1 | Np-Ag–Al | 0.036 | 10 | 1.91 |
| 2 | Np-Ag–Mg | 0.019 | 14 | 1.02 |
| 3 | Np-Pd–Al | 0.033 | 40 | 0.48 |
| 4 | Np-Au–Al | 0.028 | 15 | 1.03 |
| 5 | Np-Cu–Al | 0.017 | 8 | 1.19 |
| Entry | C4-NP/mM | CNaBH4/mM | k/min−1 |
|---|---|---|---|
| a Reactions were performed using catalyst 0.05 mmol, 4-NP and NaBH4 in 10 mL aqueous solution at room temperature. | |||
| 1 | 20 | 200 | 0.035 |
| 2 | 20 | 500 | 0.036 |
| 3 | 20 | 1000 | 0.034 |
| 4 | 20 | 2000 | 0.037 |
| 5 | 10 | 500 | 0.083 |
| 6 | 5 | 500 | 0.150 |
To better understand the behaviour of np-Ag–Al and the mechanism of the reaction, different concentration of 4-NP and NaBH4 were used in the reaction. At the same concentration of 4-NP (20 mM), changing the dosage of NaBH4 didn't significantly impact the activity. Compared with other Ag-based catalysts,27–30 even when NaBH4 was 10-fold excess, the reaction could also occur smoothly. This result confirmed the higher catalytic activity of np-Ag towards the reduction of 4-nitrophenol. As in the previous research, 100-fold excess of NaBH4 was usually used. On the other hand, when the concentration of NaBH4 (500 mM) remained unchanged, the reaction rate constant decreased with the increase of 4-NP concentration, implying that the reaction rate was in direct proportion to the concentration of 4-NP. In common, the reduction of 4-nitrophenol contains two processes, namely the decomposition of NaBH4 to produce hydrogen and the hydrogenation of aromatic nitro compounds. According to the above experimental results, it was clearly that the hydrogenation of aromatic nitro compounds was the rate limiting step (Table 2).
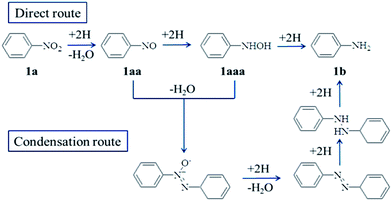
The way of the hydrogenation was further investigated, to understand the reduction mechanism. Usually, there are two accepted routes for the reduction of aromatic nitro compounds based on the electrochemical model as presented by Haber, namely direct route and condensation route.31 As shown in Scheme 1, the direct route was a stepwise hydrogenation process. During this process, the intermediate products, nitrosobenzene (1aa) and phenylhydroxylamine (1aaa), were occurred. However, in the condensation route, intermediate product was azobenzene. As the two accepted routes had different intermediate products, detecting the intermediate products would be an effective method to explore the possible mechanism. Then, the following experiment was done. When the reduction of nitrobenzene run 0.5 h, the catalyst was separated from the solution and the solution was analyzed by gas chromatography-mass spectrometer (GC-MS). Nitrobenzene (1a), nitrosobenzene (1aa) and aniline (1b) were observed, indicating np-Ag catalyzed nitroarenes reduction following the direct route. It is noteworthy, as showed in Scheme 1, the intermediate products in direct route contained nitrosobenzene (1aa) and phenylhydroxylamine (1aaa). However, in our present case, no phenylhydroxylamine (1aaa) was detected. It therefore deduced that the hydrogenation of nitrobenzene (1a) to nitrosobenzene (1aa) would be the rate limiting step.
Except the hydrogenation, electron transfer was also speculated. According to the work before, the reaction of 4-NP to 4-AP was a thermodynamically feasible process considering the BH4− (−1.33 V vs. NHE) and 4-NP (−0.76 V vs. NHE). However, it is a kinetically restricted process, (the reaction could not happen even in two days' time) in the absence of a catalyst.27,28 Usually it is believed that the electron transfer from BH4− to 4-NP was relayed by the metal surface.29,30 As the NaBH4 dissociate on the surface of np-Ag, electron would transfer on the surface of np-Ag. When the aromatic nitro compounds was adsorbed on the surface of np-Ag, the electron would further transferred from the silver surface to the nitro compounds.
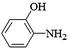
The substrate generality of the catalyst was test by using a series of aromatic nitro compounds (Table 3). Nitrobenzene without substituent group could be reduced into aniline easily (Entry 1). Aromatic nitro compounds with electron-withdrawing group and electron-donating group could also be reduced with high selectivity. For nitrophenol with the same substituent at different position, the 3-nitrophenol showed higher activity than 2-nitrophenol and 4-nitrophenol. The reason may be from the stability of the intermediate product nitrophenolate ion. As the resonance effect of 3-nitrophenol is lowest among the three nitrophenols, so the 3-NP was easier reduced. These results were in accordance with the previous literatures.32,33 Similar results were also found for the reduction of 2, 3, and 4-toluidine (Entry 5–7). The amino on the benzene ring did not affect the reduction of nitro group and the 4-phenylenediamine was achieved in 96% yield (Entry 8). Np-Ag can also be applied to reduction of nitrobenzene containing halogen or unsaturated substitutes. For example, 1-iodo-4-nitrobenzene was chemoselectively reduced to 4-iodoaniline with high selectivity (Entry 9). No dehalogenation side-product was detected. The nitroarenes with methoxycarbonyl and nitrile substitutes were reduced to methyl-4-aminobenzoate and 4-aminobenzonitrile, respectively, in high yield (Entry 10, 11) (Table 3).
| Entry | Substrate | Product | Time/min | Yield/% |
|---|---|---|---|---|
| a Reactions were performed using catalyst 0.05 mmol, aromatic nitro compounds 20 mM and NaBH4 500 mM in 10 mL aqueous solution at room temperature. | ||||
| 1 |  |
 |
90 | 99 |
| 2 | 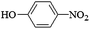 |
 |
105 | 99 |
| 3 |  |
 |
60 | 98 |
| 4 |  |
 |
60 | 99 |
| 5 |  |
 |
420 | 91 |
| 6 |  |
 |
180 | 95 |
| 7 |  |
 |
240 | 95 |
| 8 |  |
 |
360 | 96 |
| 9 |  |
 |
720 | 90 |
| 10 |  |
 |
480 | 95 |
| 11 |  |
 |
360 | 95 |
As the high stability was one of the most important properties of heterogeneous catalysts, the recycling capacity and reusability of np-Ag were also tested. After the reaction, np-Ag was separated from the reaction mixture by filtrating and the catalyst was washed by ethanol and water. Then np-Ag was reused without further purification. From Table 4, it can be seen that the catalyst could be reused even seven times without reducing the catalytic activity. Fig. 1b is the SEM image of the recovered catalyst after seven uses. Compared to the fresh one (Fig. 1a), little change in porous morphology and feature dimension was observed, but the ligament size coarsened from 30 nm before the reaction to around 40 nm. After the reaction the XPS spectrum of np-Ag was also detected, Fig. 2b. The binding energies of Ag 3d5/2 and Ag 3d3/2 were appeared at 368.5 and 374.5 eV, which implied that the surface status did not change after the reaction. To clarify whether the np-Ag catalyst had been leached into the reaction mixture or not, the reaction solution was also tested by inductivity coupled plasma (ICP-AES) analysis when the conversion of 4-NP was about 50%. The rate was lower than the detection limits (<0.02 ppm). No leaching of silver was detected. These evidences proved that np-Ag was a green and sustainable catalyst for the reduction of aromatic nitro compounds.
Conclusions
Briefly conclusion, we realized the reduction of nitroarenes with lesser amount of NaBH4 reductant under the mild reaction condition by using nanoporous silver as a high active and selective catalyst. The aromatic nitro compounds containing both electro-withdrawing and electro-donating substituents could be reduced with high yields. The catalyst can be reused several times without evident loss of its catalytic activity. As a cost-effective nanoporous material, the well reduction property exhibited by nanoporous silver here will further arouse the investigated interest of nanoporous silver in wider green organic synthesis field.Acknowledgements
Financial support from the National Science Foundation of China (21472117 and 21176144) was acknowledged.Notes and references
- Y. Ding and M. Chen, MRS Bull., 2009, 34, 569–576 CrossRef CAS.
- X. Zhang and Y. Ding, Catal. Sci. Technol., 2013, 3, 2862–2868 CAS.
- Y. Yamamoto, Tetrahedron, 2014, 70, 2305–2317 CrossRef CAS PubMed.
- C. Xu, J. Su, X. Xu, P. Liu, H. Zhao, F. Tian and Y. Ding, J. Am. Chem. Soc., 2007, 129, 42–43 CrossRef CAS PubMed.
- A. Wittstock, V. Zielasek, J. Biener, C. M. Friend and M. Bäumer, Science, 2010, 327, 319–322 CrossRef CAS PubMed.
- H. Yin, C. Zhou, C. Xu, P. Liu, X. Xu and Y. Ding, J. Phys. Chem. C, 2008, 112, 9673–9678 CAS.
- N. Asao, Y. Ishikawa, N. Hatakeyama, Y. Yamamoto Menggenbateer, M. Chen, W. Zhang and A. Inoue, Angew. Chem., Int. Ed., 2010, 49, 10093–10095 CrossRef CAS PubMed.
- M. Yan, T. Jin, Y. Ishikawa, T. Minato, T. Fujita, L. Chen, M. Bao, N. Asao, M. Chen and Y. Yamamoto, J. Am. Chem. Soc., 2012, 134, 17536–17542 CrossRef CAS PubMed.
- R. Wang, C. Wang, W. Cai and Y. Ding, Adv. Mater., 2010, 22, 1845–1848 CrossRef CAS PubMed.
- R. Wang, C. Xu, X. Bi and Y. Ding, Energy Environ. Sci., 2012, 5, 5281–5286 CAS.
- S. Tanaka, T. Kaneko, N. Asao, Y. Yamamoto, M. Chen, W. Zhang and A. Inoue, Chem. Commun., 2011, 47, 5985–5987 RSC.
- T. Kaneko, S. Tanaka, N. Asao, Y. Yamamoto, M. Chen, W. Zhang and A. Inoue, Adv. Synth. Catal., 2011, 353, 2927–2932 CrossRef CAS.
- Z. Li, S. Lin, L. Ji, Z. Zhang, X. Zhang and Y. Ding, Catal. Sci. Technol., 2014, 4, 1734–1737 CAS.
- Z. Li, C. Zhang, J. Tian, Z. Zhang, X. Zhang and Y. Ding, Catal. Commun., 2014, 53, 53–56 CrossRef CAS PubMed.
- S. Lawrence, Amines: Synthesis, properties and applications, Cambridge University Press, Cambridge, 2004 Search PubMed.
- E. Burkhardt and K. Matos, Chem. Rev., 2006, 106, 2617–2650 CrossRef CAS PubMed.
- I. Nakamula, Y. Yamanoi, T. Imaoka, K. Yamamoto and H. Nishihara, Angew. Chem., Int. Ed., 2011, 50, 5830–5833 CrossRef CAS PubMed.
- N. Sakai, K. Fujii, S. Nabeshima, R. Ikeda and T. Konakahara, Chem. Commun., 2010, 46, 3173–3175 RSC.
- R. V. Jagadeesh, G. Wienhöfer, F. A. Westerhaus, A.-E. Surkus, H. Junge, K. Junge and M. Beller, Chem.–Eur. J., 2011, 17, 14375–14379 CrossRef CAS PubMed.
- H. Wei and Y. Lu, Chem. Asian J., 2012, 7, 680–683 CrossRef CAS PubMed.
- K. Kurodaa, T. Ishidaa and M. Haruta, J. Mol. Catal. A: Chem., 2009, 298, 7–11 CrossRef PubMed.
- K. Layek, M. Lakshmi-Kantam, M. Shirai, D. Nishio-Hamane, T. Sasaki and M. Maheswaran, Green Chem., 2012, 14, 3164–3174 RSC.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2010, 26, 2885–2893 CrossRef CAS PubMed.
- Z. Zhang, C. Shao, Y. Sun, J. Mu, M. Zhang, P. Zhang, Z. Guo, P. Liang, C. Wang and Y. Liu, J. Mater. Chem., 2012, 22, 1387–1395 RSC.
- T. Kou, D. Li, C. Zhang, Z. Zhang and H. Yang, J. Mol. Catal., 2014, 382, 55–63 CrossRef CAS PubMed.
- F. Kelly and J. Johnston, ACS Appl. Mater. Interfaces, 2011, 3, 1083–1092 CAS.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2010, 26, 2885–2893 CrossRef CAS PubMed.
- J. Huang, S. Vongehr, S. Tang, H. Lu and X. Meng, J. Phys. Chem. C, 2010, 114, 15005–15010 CAS.
- P. Zhang, C. Shao, Z. Zhang, M. Zhang, J. Mu, Z. Guo and Y. Liu, Nanoscale, 2011, 3, 3357–3363 RSC.
- Z. Zhang, C. Shao, Y. Sun, J. Mu, M. Zhang, P. Zhang, Z. Guo, P. Liang, C. Wang and Y. Liu, J. Mater. Chem., 2012, 22, 1387–1395 RSC.
- F. Haber, Z. Elektrochem., 1898, 22, 506–514 Search PubMed.
- Y. Choi, H. Bae, E. Seo, S. Jang, K. H. Park and B. Kim, J. Mater. Chem., 2011, 21, 15431–15436 RSC.
- S. Jana, S. Ghosh, S. Nath, S. Pande, S. Praharaj, S. Panigrahi, S. Basu, T. Endo and T. Pal, Appl. Catal., A, 2006, 313, 41–48 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01649e |
| This journal is © The Royal Society of Chemistry 2015 |