Enhanced removal of manganese in organic-rich surface water by combined sodium hypochlorite and potassium permanganate during drinking water treatment†
Wenzheng Yu*ab,
Luiza Camposc,
Tong Shid,
Guibai Lib and
Nigel Graham*a
aDepartment of Civil and Environmental Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: w.yu@imperial.ac.uk; n.graham@imperial.ac.uk; Fax: +44 2075945934; Tel: +44 2075946121
bState Key Laboratory of Urban Water Resource and Environment (SKLUWRE), School of Municipal & Environmental Engineering, Harbin Institute of Technology, PO 73, Huanghe Road, Nangang District, Harbin, 150090, China
cDepartment of Civil, Environmental and Geomatic Engineering, University College London, Gower Street, London WC1E 6BT, UK. E-mail: l.campos.@ucl.ac.uk
dDepartment of Civil and Environmental Engineering, Zhejiang Gongshang University, Hangzhou, 310018, China
First published on 16th March 2015
Abstract
High levels of manganese (Mn) are known to occur in ground waters and some organic-rich surface waters, and are sometimes in a form (e.g. organically-bound) that is difficult to remove during conventional drinking water treatment. In this study the potential benefits of combining permanganate and chlorine prior to coagulation for Mn removal have been investigated, with particular reference to an organic-rich surface water (river Bajiang, China). The respective roles and potential synergy of permanganate and chlorine when applied together were considered by comparing the removal of Mn with the chemicals together and separately, using samples of river water and model organic-Mn solutions (humic acid and EDTA). In addition, the significance of the order of NaClO and KMnO4 dosing, and the influence of coagulant dose have been evaluated. The results have shown that the combination of the two chemicals is beneficial and synergistic. For river water containing 0.22 mg L−1 Mn, a dose of 1.76 mg L−1 NaClO reduced the half dose of the permanganate required to achieve the drinking water target concentration of 0.05 mg L−1 Mn. The addition of chlorine appears to enhance the release of bound-Mn and the subsequent conversion of Mn(II) to insoluble Mn(IV). The mechanisms responsible are believed to involve chlorine-assisted autocatalytic Mn oxidation and MnO4− recycling.
1 Introduction
Manganese concentrations in drinking water that exceed the current recommended maximum value of 0.05 mg L−1 (EU and USEPA Secondary Maximum Contaminant Level) can create problems for consumers leading to complaints about plumbing fixtures and laundry staining, discoloured water, and taste and odour.1 Also manganese exposure produces dopamine neuron degeneration and movement abnormalities.2 High levels of manganese are known to occur in ground waters and organic-rich surface waters, such as the river Bajiang (Huadu District, Guangzhou, China), where the Mn concentration is typically 0.2–0.3 mg L−1, and sometimes higher. The form of the Mn in such waters, whether inorganic or organically-bound, depends on its origin and the general water quality. Thus, in surface waters with a high concentration of organic matter the Mn may be organically-complexed and its behaviour depends on the type and concentration of the associated organic substances.3 Organically-complexed Mn is very stable, and difficult to remove during conventional drinking water treatment. The high concentration of Mn in the river Bajiang at near pH 8 is a good example, which is the basis for the research study reported here.In general, the literature describes various methods for removing Mn during drinking water treatment that have been studied and applied based on adsorption/filtration, biological treatment and chemical oxidation. With the former, some materials have been found to have a significant capability for Mn-adsorption such as Moringaoleifera seeds4 and oxidized multiwalled carbon nanotubes (MWCNTs).5 Also, Mn(II) ions can be removed effectively by filtration with modified clinoptilolite (nearly 100%)6 and with low cost crushed dolomite.7 Recently, the use of synthesized polyvinyl alcohol/3-mercaptopropyltriethoxysilane/tetraethoxysilane hybrid materials has been shown to be effective for the removal of Mn ions by adsorption.8
Biological Mn(II) removal has been the subject of a number of studies in recent years,9 and particularly by bio-filtration processes.10 Soluble manganese can be removed from water by biological processes involving manganese-oxidizing bacteria, either in situ, or in sand filters.11 A variety of full- and pilot-scale biological filters have been used for combined or simultaneous biological removal of Mn, Fe and NH4+.12 The bio-filtration can achieve Mn removal without the use of chemical agents and under natural pH conditions.13 The fungus on the media can accumulate the oxidized Mn species,14 and backwash process may remove fungus and decrease the transformation of Mn(II) to MnO2.
In comparison to the previous methods, chemical oxidation provides a rapid and practically convenient method (without changing the structure of water plant) for manganese removal and the application of potassium permanganate oxidation (at 6.5 < pH < 8.5) followed by flocculation, settling and filtration is a common treatment scheme,15 especially for large-scale water treatment plants.16 The insoluble products of permanganate reduction and Mn(II) oxidation are Mn(III) and Mn(IV) oxides, MnOx(s),17 and these can have a catalytic effect on the adsorption and removal of soluble Mn(II) from influent waters.18 Also the manganese can be removed by ozonation19 and by impregnated activated carbon coated by permanganate.20 However, where Mn(II) is present in relatively high concentrations, and/or in a complexed form, large doses of permanganate may be required which may lead to residual Mn concentrations that exceed the regulated level in treated waters. Alternatively, the use of chlorine combined with the catalytic action of MnOx(s) oxides can assist Mn(II) removal by oxidizing adsorbed Mn(II) to more stable MnOx(s).21 While the removal of various levels of iron and manganese (higher than 80%) from lake water has been reported to be high using an in-line pre-chlorination step.22 Various studies (e.g.23) have shown that the oxidation of dissolved manganese (Mn(II)) during chlorination is a relatively slow process which may lead to high residual Mn(II) in treated drinking waters (although the rate may be enhanced in bromide-containing waters).
To-date, no previously reported studies have considered the potential advantages of combining permanganate and chlorine to enhance the removal efficiency of Mn. Thus, in this study the performance and mechanisms of combining permanganate oxidation with pre-chlorination for Mn removal are investigated, with particular focus on the problem of Mn in organic-rich surface water sources, as represented by the case of the river Bajiang. Pre-chlorination of surface waters is often used as a means of reducing ammonia and for other treatment benefits at doses that do not cause significant halogenated by-product formation, but by itself chlorination is unable to oxidize Mn effectively at pH < 9.24 Thus, the respective roles and potential synergy of permanganate and chlorine are considered in this study by comparing the removal of Mn with the chemicals together and separately, focusing on samples of river Bajiang and model organic-Mn solutions (humic acid and EDTA). The results have shown that the combination of the two chemicals is beneficial and synergistic and this is described in detail as follows.
2 Materials and methods
2.1 Chemicals and test solutions
Polyaluminum chloride (PACl, 1.5 basicity) was used as the coagulant in this study, purchased from Guangzhou Jiequan, China. Stock KMnO4 was prepared as 1000 mg L−1; stock NaClO was prepared as 1000 mg L−1 every day and was considered stable for one day.A bulk sample of raw water (as experimental water here) was obtained from the river Bajiang, Guangzhou, China, and stored at 4 °C during the period of laboratory testing. Samples were allowed to come to room temperature prior to experiments. Relevant values of the raw water quality are as follows: Mn 0.22 ± 0.03 mg L−1, Fe 0.03 ± 0.01 mg L−1, TOC 6.5 ± 0.4 mg L−1, trihalomethanes (THMs) formation potential 1.54 ± 0.28 mg L−1, turbidity 14.3 ± 1.5 NTU, colour 30 ± 2 oH, pH 7.84 ± 0.13, NH4+–N 1.15 ± 0.12 mg L−1, alkalinity 148 ± 23 mg L−1 (calculated as CaCO3). There was little bromide in the water (below 10 μg L−1, the detection limit of bromide concentration by ion chromatography).
For the tests involving the preparation of solutions of EDTA–Mn and humic acid–Mn, an appropriate quantity of either EDTA (ethylenediaminetetraacetic acid, Sigma, USA) or humic acid (HA, sodium salt, Aldrich, USA) was added to deionised (DI) water to give a concentration of 5 mg L−1 to represent the complexion of Mn(II) with EDTA and humic acid (it is confirmed in section 3.5 that there is little complex between HA and Mn2+ and there is complex between EDTA and Mn2+); neutral pH was obtained by the addition of 5 mM NaHCO3 and by adding NaOH or HCl. Subsequently, 1 mg L−1 Mn2+ (as MnCl2) was added to the respective solutions and mixed continuously for 24 h, which was used as model waters for exploring mechanism (the molar ratio of EDTA and Mn2+ was near 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is stoichiometry of the EDTA–Mn complexes). EDTA was chosen here as it had been found in raw water supplies,25 and it can represent the organic matter, which can complex with the Mn in the water. The model water was used in section 3.5 only.
1, which is stoichiometry of the EDTA–Mn complexes). EDTA was chosen here as it had been found in raw water supplies,25 and it can represent the organic matter, which can complex with the Mn in the water. The model water was used in section 3.5 only.
2.2 Treatment experiments
The treatment process for Mn, as well as other contaminants, employed two 0.5 L h−1 mini-pilot scale water plants in parallel (Fig. S1,† only one was demonstrated), which comprised mixing tanks, flocculation tanks, a sedimentation tank, and a sand filtration column, in order to simulate the real water treatment plant. NaClO (0–4.4 mg L−1), KMnO4 (0–5 mg L−1) and PACl (dosed at 7.2 mg L−1, except where otherwise stated) were continuously added to the raw water via the mixing tanks, and the mode of addition of oxidants (together or separately) was varied as part of the study. The rapid mix speed was 200 rpm (180 s−1) in the mixing tanks each with a hydraulic retention time (HRT) of 1 min, and 50 rpm (23 s−1) in the three flocculation tanks, each with a HRT of 5 min. Subsequently the coagulated water passed into the sedimentation tank for flocs to settle (HRT 15 min) and then to the sand filtration column (sand size: 0.5–0.8 mm) with a 5 min real HRT (pore volume in the sand tank divided by the flow rate). The total HRT of the system was 40 min, and each experiment was conducted for 2 h to ensure the results were representative of the process performance.Following the tests with the raw water additional tests were carried out with solutions of EDTA–Mn and humic acid–Mn using the same mini-pilot scale system (section 3.5). Each solution was prepared as described in section 2.1. All treatment tests were carried out for three replicates at 25 ± 2 °C.
2.3 Analytical methods
Turbidity was determined by a WTW TURB555IR turbidimeter. Residual manganese and aluminum after sand filtration were measured by inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 7300 DV, PerkinElmer, USA). Dissolved organic carbon (DOC) was determined with a total organic carbon (TOC) analyzer (TOC-VCPH, Shimadzu, Japan). The colour in the water was measured by the chromium – cobalt colorimetry method, and the UV-visible absorbance (between 190 nm and 800 nm, by pre-experiment) of 0.45 μm filtered solutions was determined by spectrophotometry (U-3010, Hitachi High Technologies Co., Japan). Active chlorine was determined by spectrophotometry using N,N-diethyl-1,4-phenylenediamine (DPD). The concentration of KMnO4 was measured through visible absorbance at 535 nm.263 Results
A first series of tests were undertaken to establish the effect of permanganate treatment on Mn removal, with and without the addition of NaClO, for a range of NaClO and KMnO4 doses. For these tests the coagulation dosage of PACl, 7.2 mg L−1, was chosen to be the same as the dosage used at the Bajiang water treatment plant (and confirmed in the mini-pilot scale tests – section 3.4). No pH correction was made, so the solution pH decreased slightly from 7.9 to 7.5 after PACl addition. Subsequent tests were carried out to consider the significance of the order of NaClO and KMnO4 addition, and the influence of coagulant dose. In order to provide further insight into the reaction mechanism, further tests were conducted using model Mn-organic solutions corresponding to weak and strong ligands in section 3.5 (Mn–humic acid and Mn–EDTA respectively).3.1 Effect of NaClO dose on the removal of Mn
The effect of different dosages of NaClO (0–4.4 mg L−1) on the removal of Mn was explored with an applied KMnO4 dosage of either 0 or 1.5 mg L−1 (Fig. 1). In these tests, after NaClO and KMnO4 were added simultaneously, PACl was added to the solution 1 min later. In the absence of both NaClO and KMnO4 there was no measurable change in the Mn concentration indicating that coagulation had no impact on the inorganic and organic species of Mn presenting, although part of organic matter was removed (35%). This is consistent with other work which reported that Mn-bound organic matter was of low molecular (MW) weight substances (MW < 2000),27 as larger MW organic matter was much easier to be removed by coagulation process. With the addition of NaClO alone (no KMnO4) there was a minor (0–15%), increasing reduction in Mn with increasing NaClO dose (Fig. 1) indicating some release and oxidation of organically-bound Mn. However, with the addition of 1.5 mg L−1 KMnO4 the effluent Mn concentration (0.035–0.115 mg L−1) was much lower than without KMnO4 (0.18–0.22 mg L−1) for all NaClO doses. When NaClO doses equal or lower than 0.44 mg L−1, no synergistic effect is observed at these conditions and the reduction of dissolved Mn(II) being mainly due to KMnO4. This result may be because of the reaction of NH4+–N and NaClO (k ∼ 104 M−1 s−128 at pH 7). After that, it can be seen that the reduction in effluent Mn concentration increased substantially up to a NaClO dose of 1.76 mg L−1 (50% higher removal) with 1.5 mg L−1 KMnO4, which meant that there was synergetic effect for NaClO and KMnO4.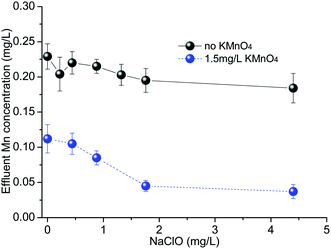 | ||
| Fig. 1 The effect of sodium hypochlorite dosage on the effluent manganese concentration in Bajiang river water (NaClO and KMnO4 were added simultaneously 1 min prior to 7.2 mg L−1 PACl). | ||
3.2 Effect of KMnO4 dose on the removal of Mn
The influence of KMnO4 dose on the removal of Mn was investigated both without, and with, NaClO (0 mg L−1 or 1.76 mg L−1, respectively) (Fig. 2). For the former case, the theoretical, stoichiometric molar ratio for the oxidation of free Mn2+ to MnO2 by permanganate is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Mn2+
2 (Mn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4), which means that a minimum KMnO4 dose of 0.42 mg L−1 would be required to remove all Mn2+ in the raw water if the Mn(II) was freely available (unbound). It can be seen in Fig. 2 that the reduction in Mn(II) concentration was only about 10% for a KMnO4 dose of 0.42 mg L−1 with nearly 0 mg L−1 residual KMnO4. Since the reactivity of non-complex Mn2+ with permanganate is much greater (k ∼ 105 M−1 s−129) than permanganate with organic substrates (k < 102 M−1 s−130), the low Mn removal from the raw water at a low, but stoichiometric, KMnO4 dose (∼0.5 mg L−1) is consistent with the Mn being present in an organically-bound form. No detected residual KMnO4 further confirmed this result. At higher doses of KMnO4 it is assumed that there is greater reactivity with the organic matter, some of which is related to the severing of Mn-organic bonds, thereby leading to a greater removal of Mn. Thus, a KMnO4 dose of 3 mg L−1 achieved a residual Mn concentration (after coagulation/filtration) of 0.05 mg L−1, representing a reduction of ∼77%.
KMnO4), which means that a minimum KMnO4 dose of 0.42 mg L−1 would be required to remove all Mn2+ in the raw water if the Mn(II) was freely available (unbound). It can be seen in Fig. 2 that the reduction in Mn(II) concentration was only about 10% for a KMnO4 dose of 0.42 mg L−1 with nearly 0 mg L−1 residual KMnO4. Since the reactivity of non-complex Mn2+ with permanganate is much greater (k ∼ 105 M−1 s−129) than permanganate with organic substrates (k < 102 M−1 s−130), the low Mn removal from the raw water at a low, but stoichiometric, KMnO4 dose (∼0.5 mg L−1) is consistent with the Mn being present in an organically-bound form. No detected residual KMnO4 further confirmed this result. At higher doses of KMnO4 it is assumed that there is greater reactivity with the organic matter, some of which is related to the severing of Mn-organic bonds, thereby leading to a greater removal of Mn. Thus, a KMnO4 dose of 3 mg L−1 achieved a residual Mn concentration (after coagulation/filtration) of 0.05 mg L−1, representing a reduction of ∼77%.
For the tests with NaClO the dose of 1.76 mg L−1 was chosen since this corresponded to the practical optimal concentration found in the previous tests that achieved a low level of Mn with 1.5 mg L−1 KMnO4 (see Fig. 1). This is illustrated by the much higher dosage of KMnO4 needed to meet the current water quality regulation for Mn of 0.05 mg L−1, if no NaClO is added, compared to that with NaClO (nearly two times). Whether NaClO was present or not, the effluent concentration of Mn decreased to lower than 0.05 mg L−1 as the dosage of KMnO4 increased up to 5 mg L−1. However, the effluent Mn concentration was much lower (0.02–0.05 mg L−1 less) for the combination of KMnO4 with 1.76 mg L−1 NaClO than without NaClO. Clearly, these results show the benefit of combining NaClO with KMnO4 on the overall reduction of Mn, and there appeared to be a synergistic effect between the two chemicals (maximum at approximately 1.5 mg L−1 KMnO4/1.76 mg L−1 NaClO doses) which will be discussed in section 4. Furthermore, although there was little further reduction in Mn at KMnO4 doses >3 mg L−1, indicating that a fraction of the organic-complexed Mn cannot be removed (or because of the presence of unfiltered colloidal MnOx), the effluent concentration of Mn was lower at all KMnO4 doses with the combined NaClO/KMnO4 compared to KMnO4 alone.
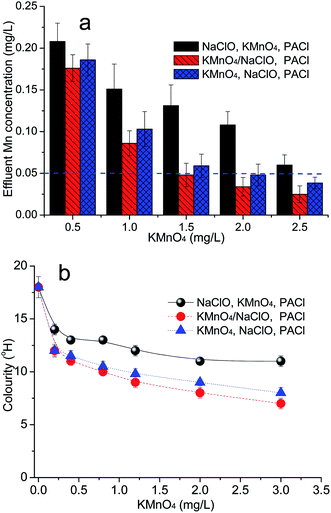
3.3 Effect of chemical dosing sequence on the removal of Mn
In these tests a comparison was made between the dosing arrangement used previously (i.e. NaClO was added before, together and later than KMnO4, followed by PACl added 1 min later) (Table S1†), on the removal of Mn. The results showed that when NaClO and KMnO4 were added together before PACl, the effluent Mn concentration for all KMnO4 dosages was much lower (around 50%) than the other two arrangements of adding two oxidants separately, especially delaying the KMnO4 addition (after NaClO) (Fig. 3a). It can be seen from Fig. 3a that the mixed dosing arrangement enabled the required Mn maximum concentration (0.05 mg L−1) to be achieved with 1.5 mg L−1 KMnO4, whereas for the KMnO4–NaClO arrangement it needs 2.0 mg L−1 and for NaClO–KMnO4 arrangement even the highest KMnO4 dose of 2.5 mg L−1 could not achieve an effluent Mn concentration ≤0.05 mg L−1.A similar trend of behavior was found for solution colour (Fig. 3b). The colour arises from the presence of dissolved organic substances, some of which are associated with Mn. It was clear that the co-addition of NaClO and KMnO4 prior to coagulation was much more effective (10–30%) in reducing the colour than adding two oxidants separately before PACl coagulation, suggesting a greater oxidation effect. However, the removal by adding KMnO4 and then NaClO was much close to the one by adding KMnO4 and NaClO together, as the residual Mn concentration of these two arrangements was same. Nano-MnOx particles formed after adding KMnO4 to the raw water and aggregation between these nanoparticles may occur, which would decrease the catalysis of NaClO when NaClO was added later. This will be discussed subsequently.
3.4 Effect of PACl dosage on the removal of Mn
The coagulation process is of major importance for the separation and removal of colloidal MnOx(s) particles formed by the pre-oxidation. The influence of PACl dose on the treatment performance, in terms of the effluent Mn concentration and turbidity after settling and filtration, is shown in Fig. 4. As expected, dose regions corresponding to no-, low- and high-coagulation were evident which are characteristic of ‘sweep flocculation’ by insoluble Al-hydrolysis species. High coagulation efficiency was observed for PACl doses >4.8 mg L−1 and high dose of PACl induced higher removal of Mn, such as 7.2 mg L−1, which is applied at full-scale water plant. The result meant the addition of permanganate could form nano-colloidal particles with Mn(II) in the water,31 and the coagulation process is of great importance on the removal of these new formed MnOx particles. A close relationship was evident between residual Mn and turbidity which confirmed that the residual Mn was in the form of colloidal particles. | ||
| Fig. 4 Effect of PACl dosage on the effluent concentration of manganese and turbidity in Bajiang river water (1.76 mg L−1 NaClO and 1.5 mg L−1 KMnO4 were added simultaneously 1 min prior to PACl). | ||
3.5 Model Mn-organic solutions
In order to further examine the nature of the reactions between KMnO4 and organically-bound Mn, with and without NaClO, model Mn-organic solutions corresponding to weak and strong complexes (Mn–humic acid and Mn–EDTA respectively) were considered. For the case of Mn–humic acid, Fig. 5a shows the removal of Mn by KMnO4 oxidation and PACl coagulation. The dose of KMnO4 was varied from 0 to 3.84 mg L−1 and the PACl dose was maintained at an optimal value of 2.7 mg L−1 (see Fig. 5b). In the absence of KMnO4 (0 mg L−1) there was no measurable change in the Mn concentration (1 mg L−1) despite good coagulation of the humic material (great decrease of UV254 value, which is in line with humic concentration), indicating that the humic acid had little complexed Mn(II) during the preparation of the model water. With the addition of KMnO4 the concentration of residual Mn reduced systematically with increasing KMnO4 dose and was almost zero at a KMnO4 dose of 1.92 mg L−1 (Fig. 5a), corresponding almost exactly with the theoretical dose required for the oxidation of free Mn2+ to MnO2 by permanganate. This is consistent with the much faster reaction of permanganate with Mn2+ than with organic substrates, as mentioned previously. Coagulation is needed to separate the MnO2 formed by the permanganate, as shown in Fig. 5b (i.e. no Mn removal at 0 mg L−1 PACl), and a PACl dose of 2.70 mg L−1 was found to be the optimal dose.At KMnO4 doses greater than 1.92 mg L−1, the excess permanganate (i.e. [KMnO4]− 1.92 mg L−1) is unavailable to react with the humic acid in solution quickly. This leads to high residual Mn concentration with increasing KMnO4 dose, when it was higher than 1.92 mg L−1. The equivalent of ∼40% of the Mn from the excess permanganate was not removed at a KMnO4 dose of 3.84 mg L−1 (Fig. 5a).
In contrast, free Mn(II) ions readily complex with EDTA,32 and the respective reactions of Mn–EDTA with 1 mg L−1 NaClO and 1.92 mg L−1 KMnO4, separately and together, and with coagulation (at different PACl dosages) are shown in Fig. 5c. With NaClO alone (1 mg L−1), there was virtually no removal of Mn. KMnO4 can oxidize/react with EDTA, but the kinetics are very slow33 and no significant oxidation was detectible in 60 min (Fig. S2a†). A significant reaction was evident with KMnO4 which achieved a reduction in Mn that was approximately 65% of the total Mn in solution (2 mg L−1; from 1 mg L−1 Mn as EDTA–complexed Mn and 1 mg L−1 Mn as added KMnO4) with around 7 mg L−1 PACl (Fig. 5c). Thus, the results indicate the capability of KMnO4 to attack the EDTA–Mn bond and release the Mn from the EDTA complex. However, the combination of NaClO and KMnO4 produced a degree of Mn removal that was much greater than with KMnO4 alone (Fig. 5c). From the relative changes in residual Mn it is clear that the addition of NaClO provides a synergistic benefit to the KMnO4 reaction. Since the addition of NaClO had no significant impact on the oxidation of EDTA by KMnO4 (See Fig. S2b†), it can be concluded that the synergistic effect must be related to the severing of the Mn–EDTA bond.
Information of the variation of KMnO4 and MnOx was obtained from UV-visible spectroscopy of the reactions, as summarized in Fig. 6. Changes in the UV-visible light absorbance of solutions were followed for reaction times up to 1 h. As indicated previously, there was no evidence of any reaction between EDTA–Mn and NaClO alone over 1 h (Fig. 6a). However, for the reaction between EDTA–Mn and KMnO4 alone, or KMnO4 with NaClO, broad spectra (200–600 nm) were evident representing the combined absorbance of KMnO4 (around 535 nm) and colloidal MnOx (around 330 nm) (Fig. 6b and c, respectively).34 As a reference, the spectrum for MnO2 was produced by the Mn2+/KMnO4 reaction which indicated an absorbance peak at 300–400 nm, as shown in Fig. 6d; this peak is consistent with other studies of MnO2.9,35 It is clear that the extent of MnO2 produced and KMnO4 decreased in the reactions with KMnO4 alone or NaClO and KMnO4 together, which were time dependent (Fig. 6b and c) and rapid, with significant changes occurring after only 1 min. However, the relative rate of reaction in the two cases was different. This can be seen by comparing Fig. 6b with 6c, which shows that the absorbance spectrum for the NaClO and KMnO4 together at 10 min was the same as that for the KMnO4 alone at 30 min, and similarly at 15 min and 60 min, respectively. These results demonstrate that the reaction was much faster for NaClO and KMnO4 together than KMnO4 alone, and Fig. 6d clearly shows the relative superiority of NaClO and KMnO4 after 15 min oxidation, particularly in terms of the MnO2 peak.
 | ||
| Fig. 6 Effect of different oxidant combinations on the EDTA–Mn solution as indicated by UV/visible absorption in model waters (same reaction conditions as Fig. 5, but without coagulant and finished in bottled experiment): (a) NaClO; (b) KMnO4; (c) NaClO + KMnO4; (d) EDTA–Mn or Mn2+ with different oxidant combinations after 15 min. | ||
4 Discussions
The removal of manganese from surface and ground waters is often a requirement during drinking water treatment in order to meet national water quality regulations. In cases where the manganese speciation is either inorganic or bound to low molecular weight organic matter, the Mn is poorly removed by conventional coagulation and filtration processes, and for the river Bajiang this has led to a long period of non-compliance with the water treatment objective of achieving Mn < 0.05 mg L−1. Pre-treatment with chlorine is capable of oxidizing Mn(II) but is not efficient at pH < 9 (k ∼ 6.4 × 10−4 M−1 s−1 at pH 8 (ref. 36)) and elevated chloride doses may lead to undesirable levels of halogenated by-product compounds. In contrast, permanganate has been shown to be an effective oxidant in circumneutral conditions and in this study a relatively high KMnO4 dose of 3 mg L−1, prior to coagulation and filtration, was able to achieve 0.05 mg L−1 Mn in the treated water from the original river water concentration of 0.22 mg L−1 Mn. However, with a simultaneous dose of chlorine (1.76 mg L−1) the permanganate dose required was approximately halved (1.5 mg L−1) and the combination of the two chemicals appeared to be synergistic. A similar behavior was found for the model solution containing the Mn–EDTA complex. The results showed that the combination of chlorine and permanganate was more effective than permanganate alone in separating the Mn from the complex.Three possible reasons for the advantageous and synergistic combination of chlorine and permanganate in the treatment of organically-bound Mn are proposed as follows:
(i) Reduction in permanganate oxidation demand by chlorine;
(ii) Recycling of permanganate by chlorine oxidation of MnO2;
(iii) Enhanced autocatalytic process of Mn oxidation/precipitation.37
The first of these effects is that chlorine, as a co-oxidant, can lower the permanganate oxidation demand of the water leading to a relatively greater KMnO4 concentration available to react with the Mn species, both for releasing the bound Mn and for oxidizing the Mn to solid phase MnOx. Given the estimated oxidation strength (reduction potential) of chlorine under the test conditions (EOCl−/Cl− ∼ +1.27 V, pH 7.8), it is expected that some degree of general solute oxidation occurs, including the release of bound Mn as indicated in Fig. 1. Thus, the two chemical oxidants (chloride and KMnO4) can be considered as complementary. However, the marked difference in treatment performance observed in this study when the chlorine was added either together with, or prior to, the KMnO4 (Fig. 3) showed that other reaction phenomena are also involved.
The second reason for the beneficial effect of chlorine is the possible in situ recycling of MnO4− ions by the chlorine oxidation of MnO2 (viz. MnO2(s) + 4OH− → MnO4− + 2H2O + 3e−), with the latter formed by KMnO4 reduction via solute oxidation reactions (MnO4− → MnO2). Thermodynamically, this is feasible since the reduction potential of hypochlorite in the solution conditions (EOCl−/Cl− ∼ +1.27 V, pH 7.8) exceeds the oxidation potential of MnO2 (EMnO2/MnO4− ∼ −0.99 V, pH 7.8), but the kinetics of the reaction are unclear. If the kinetics of the recycling of MnO4− ions were favourable then this would enhance the MnO4− concentration during the reaction period (while sufficient chlorine is present) giving two benefits: a greater release of bound-Mn and conversion of the released Mn2+ to MnOx(s).
The third effect relates to the Mn autocatalysis process whereby Mn2+ ions released from organo-Mn compounds readily adsorb on the surfaces of in situ formed Mn oxide surfaces (MnOx(s)) where they subsequently oxidize to MnO2. The presence of chlorine in solution greatly enhances the rate at which the adsorbed Mn(II) can be oxidized to Mn(IV) (‘heterogeneous oxidation’), and this in turn accelerates the further adsorption of Mn(II)36,37 because of the increased quantity, and surface area of MnO2. Therefore, the new formed MnO2 nanoparticles with NaClO can remove more Mn(II) in the raw water as it had greater surface area when KMnO4 was added together with NaClO (Fig. 3).
Both the second and third effects correspond to the observed synergistic behavior since they depend on the formation of MnOx(s), which is only poorly formed by chlorine alone. The magnitude of the synergy will be dependent on the concentration and duration of chlorine present during the reactions, as demonstrated by the poorer performance observed when chlorine was added in advance of permanganate (Fig. 3), where chlorine consumption by reactive substrates in solution leads to a lower chlorine concentration than when it is added simultaneously with permanganate. The greater reduction of solution colour when permanganate and chlorine were added simultaneously may be explained by a greater oxidation because of the maintenance of higher permanganate concentrations through the effect of in situ recycling of MnO4− ions by the chlorine. In addition, the greater reduction of colour may be due to enhanced adsorption of organic chromophores on to MnOx(s), and the more efficient removal of the MnOx(s) by the coagulation/filtration.
5 Conclusions
In this study the potential benefits of combining permanganate and chlorine prior to coagulation for Mn removal have been investigated, with particular focus on the problem of organically-bound Mn. The principal conclusions care as follows:• The simultaneous addition of NaClO and KMnO4 enhanced the overall removal of Mn compared to KMnO4 alone.
• The sequence of NaClO and KMnO4 addition was found to affect overall the Mn reduction; simultaneous addition of the chemicals was superior to adding NaClO before KMnO4.
• Whilst KMnO4 is able to break Mn-organic bonds, the combination of NaClO and KMnO4 was found to enhance this effect synergistically.
• The greater performance of combined NaClO and KMnO4 is believed to occur via mechanisms involving chlorine-assisted autocatalytic Mn oxidation and MnO4− recycling.
Acknowledgements
The authors wish to acknowledge the assistance of the Guangzhou-Huadu Water Company, China. This research was supported by a Marie Curie International Incoming Fellowship within the 7th European Community Framework Programme for Dr Wenzheng Yu (FP7-PEOPLE-2012-IIF-328867).References
- P. Kohl and S. Medlar, Awwarf Report 91147, 2007.
- T. R. Guilarte, Cienc Saude Coletiva, 2011, 16, 4519 CrossRef.
- E. O. Sommerfeld, Iron and Manganese Removal Handbook, Denver, CO, AWWA, 1999 Search PubMed.
- T. L. Marques, V. N. Alves, L. M. Coelho and N. M. M. Coelho, BioResources, 2013, 8, 2738 CrossRef.
- P. Ganesan, R. Kamaraj, G. Sozhan and S. Vasudevan, Environ. Sci. Pollut. Res., 2013, 20, 987 CrossRef CAS PubMed.
- Y. I. Tarasevich, V. V. Goncharuk, V. E. Polyakov, D. A. Krysenko, Z. G. Ivanova, E. V. Aksenenko and M. Y. Tryfonova, J. Ind. Eng. Chem., 2012, 18, 1438 CrossRef CAS.
- H. A. Aziz and P. G. Smith, Water Res., 1996, 30, 489 CrossRef CAS.
- N. Georgieva, R. Bryaskova, N. Lazarova and R. Racheva, Biotechnol. Biotechnol. Equip., 2013, 27, 4078 CrossRef CAS.
- C. N. Butterfield, A. V. Soldatova, S. W. Lee, T. G. Spiro and B. M. Tebo, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11731 CrossRef CAS PubMed.
- (a) M. S. Burger, S. S. Mercer, G. D. Shupe and G. A. Gagnon, Water Res., 2008, 42, 4733 CrossRef CAS PubMed; (b) I. A. Katsoyiannis and A. I. Zouboulis, Water Res., 2004, 38, 1922 CrossRef CAS PubMed; (c) S. Y. Qin, F. Ma, P. Huang and J. X. Yang, Desalination, 2009, 245, 183 CrossRef CAS.
- (a) A. M. Gounot, FEMS Microbiol. Rev., 1994, 14, 339 CrossRef CAS PubMed; (b) B. M. Tebo, J. R. Bargar, B. G. Clement, G. J. Dick, K. J. Murray, D. Parker, R. Verity and S. M. Webb, Annu. Rev. Earth Planet. Sci., 2004, 32, 287 CrossRef CAS; (c) V. W. Hoyland, W. R. Knocke, J. O. Falkinham, A. Pruden and G. Singh, Water Res., 2014, 66, 31 CrossRef CAS PubMed; (d) J. R. Bargar, B. M. Tebo and J. E. Villinski, Geochim. Cosmochim. Acta, 2000, 64, 2775 CrossRef CAS.
- (a) A. G. Tekerlekopoulou, S. Pavlou and D. V. Vayenas, J. Chem. Technol. Biotechnol., 2013, 88, 751 CrossRef CAS; (b) M. Han, Z. W. Zhao, W. Gao and F. Y. Cui, Bioresour. Technol., 2013, 145, 17 CrossRef CAS PubMed; (c) J. Vandenabeele, M. Vandewoestyne, F. Houwen, R. Germonpre, D. Vandesande and W. Verstraete, Microbial Ecology, 1995, 29, 83 CrossRef CAS PubMed.
- (a) V. A. Pacini, A. M. Ingallinella and G. Sanguinetti, Water Res., 2005, 39, 4463 CrossRef CAS PubMed; (b) A. G. Tekerlekopoulou and D. V. Vayenas, Biochem. Eng. J., 2008, 39, 215 CrossRef CAS; (c) P. Berbenni, A. Pollice, R. Canziani, L. Stabile and F. Nobili, Bioresour. Technol., 2000, 74, 109 CrossRef CAS.
- K. Sasaki, H. Konno, M. Endo and K. Takano, Biotechnol. Bioeng., 2004, 85, 489 CrossRef CAS PubMed.
- (a) P. Roccaro, C. Barone, G. Mancini and F. G. A. Vagliasindi, Desalination, 2007, 210, 205 CrossRef CAS; (b) J. E. Vanbenschoten, L. Wei and W. R. Knocke, Environ. Sci. Technol., 1992, 26, 1327 CrossRef CAS.
- R. Raveendran, B. Chatelier and K. Williams, Water Sci. Technol.: Water Supply, 2002, 2, 173 CAS.
- Q. W. Li, G. Luo, J. Li and X. Xia, J. Mater. Process. Technol., 2003, 137, 25 CrossRef CAS.
- (a) G. Hamilton, B. Chiswell, J. Terry, D. Dixon and L. Sly, J. Water Supply: Res. Technol.--AQUA, 2013, 62, 417 CrossRef CAS; (b) M. M. Michel and L. Kiedrynska, Przem. Chem., 2012, 91, 1416 CAS; (c) J. Jez-Walkowiak and Z. Dymaczewski, J. Water Supply: Res. Technol.--AQUA, 2012, 61, 364 CrossRef CAS; (d) T. Ormanci, G. T. Demirkol, I. M. Aydin and N. Tufekci, Desalin. Water Treat., 2013, 51, 2225 CrossRef CAS.
- K. F. Mcknight, M. Carlson, P. Fortin and C. Ziesemer, Ozone: Sci. Eng., 1993, 15, 331 CrossRef CAS.
- E. Okoniewska, J. Lach, M. Kacprzak and E. Neczaj, Desalination, 2007, 206, 251 CrossRef CAS.
- (a) J. M. Cerrato, W. R. Knocke, M. F. Hochella, A. M. Dietrich, A. Jones and T. F. Cromer, Environ. Sci. Technol., 2011, 45, 10068 CrossRef CAS PubMed; (b) W. R. Knocke, L. Zuravnsky, J. C. Little and J. E. Tobiason, J. - Am. Water Works Assoc., 2010, 102, 64 CAS.
- K. H. Choo, H. Lee and S. J. Choi, J. Membr. Sci., 2005, 267, 18 CrossRef CAS.
- S. Allard, L. Fouche, J. Dick, A. Heitz and U. von Gunten, Environ. Sci. Technol., 2013, 47, 8716 CrossRef CAS PubMed.
- W. Stumm and J. J. Morgan, Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, Wiley, New York, NY, 1996 Search PubMed.
- E. Gilbert and S. Hoffmannglewe, Water Res., 1990, 24, 39 CrossRef CAS.
- J. F. Perez-Benito and J. Ferrando, J. Phys. Chem. B, 2014, 118, 14949 CAS.
- R. Munter, P. Overbeck and J. Sutt, Ozone: Sci. Eng., 2008, 30, 73 CrossRef CAS.
- M. Deborde and U. von Gunten, Water Res., 2008, 42, 13 CrossRef CAS PubMed.
- J. E. Vanbenschoten, L. Wei and W. R. Knocke, Environ. Sci. Technol., 1992, 26, 1327 CrossRef CAS.
- R. H. Waldemer and P. G. Tratnyek, Environ. Sci. Technol., 2006, 40, 1055 CrossRef CAS PubMed.
- F. Freeman and J. C. Kappos, J. Am. Chem. Soc., 1985, 107, 6628 CrossRef CAS.
- R. N. Collins, B. C. Onisko, M. J. McLaughlin and G. Merrington, Environ. Sci. Technol., 2001, 35, 2589 CrossRef CAS PubMed.
- R. N. Bose, C. Keane, A. Xidis, J. W. Reed, R. M. Li, H. Tu and P. L. Hamlet, Inorg. Chem., 1991, 30, 2638 CrossRef CAS.
- J. Jiang, S. Y. Pang and J. Ma, Environ. Sci. Technol., 2010, 44, 4270 CrossRef CAS PubMed.
- M. Altaf and D. Jaganyi, J. Dispersion Sci. Technol., 2013, 34, 1481 CrossRef CAS.
- O. J. Hao, A. P. Davis and P. H. Chang, J. Environ. Eng., 1991, 117, 359 CrossRef CAS.
- M. A. Kessick and J. J. Morgan, Environ. Sci. Technol., 1975, 9, 157 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01643f |
| This journal is © The Royal Society of Chemistry 2015 |