Microorganism-based monodisperse microcapsules: encapsulation of the fungicide tebuconazole and its controlled release properties†
Bo Zhanga,
Teng Zhangb,
Quanxi Wangb and
Tianrui Ren *b
*b
aState Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering/Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, 550025, P. R. China
bThe Key Laboratory of Resource Chemistry of Ministry of Education, The Development Centre of Plant Germplasm Resources, College of Life and Environmental Science, Shanghai Normal University, 100 Guilin Road, Shanghai, 200234, P. R. China. E-mail: trren@shnu.edu.cn; Fax: +86 21 64328850; Tel: +86 21 64328850
First published on 4th March 2015
Abstract
The design of an ideal monodisperse microcapsulation system, which could meet the need for prolonged and better control of drug administration, is a great challenge. Herein cyanobacteria cells served as a natural environmentally-friendly wall material to encapsulate the fungicide tebuconazole (TEB), and then urea–formaldehyde (UF) resins were automatically coated on it via electrostatic interactions. By this means, monodisperse TEB–PCC@UF microcapsules were achieved, which not only can effectively control the drug release rate but also depress the initial “burst effect” to some degree. A bioactivity experiment showed that TEB–PCC@UF microcapsules authentically prolonged the antifungal effects, and were very efficacious in controlling wheat powdery mildew compared with the commercial formulation.
Introduction
Wheat is the most widely cultivated and important food crop in the world as a staple crop for about 35% of the human population.1 Wheat powdery mildew, caused by Blumeria graminis f. sp. Tritici, has been recognized as the main and widespread disease of wheat in the growing areas leading to a significant yield decrease and economic loss worldwide.2–4 The use of pesticides is essential for preventing and controlling it. Tebuconazole (TEB) as a broad-spectrum, high-efficiency, and low-toxicity triazole systemic fungicide is effective against rust, powdery mildew, net blotch, root rot, scab, smut and seed-borne diseases on a variety of cereal crops.5 The most common formulations of TEB are emulsifiable concentrates and wettable powders due to its inferior water-solubility (0.032 g L−1 at 20 °C). There are still serious problems in these formulations due to the immediate release of the active ingredients, which greatly reduce TEB efficacy. Therefore, excessive quantities of TEB are needed to compensate such losses, also resulting in a severe economic loss. Meanwhile, it is harmful to human health as well as the environment. Thus, how to enhance the efficacy of TEB and minimize its environmental impacts is always an important issue. This problem has stimulated interesting in developing new formulations to improve them. Especially, microencapsulation formulation is one choice for it.Microencapsulation of pesticides is a versatile technology for controlled drug release in which numerous synthetic6–10 and natural materials11–17 have been widely employed as pesticide carriers. Many achievements have been made in the field of pesticide encapsulation for controlled release, and encapsulated pesticides have exhibited controlled-release properties, provided enhanced efficacy and reduced the impacts.6–17 However, these single-walled microcapsules cannot control the release of the core materials effectively, and lead to high initial burst release because they were often made of thin polymeric membranes. The double-walled microspheres with a drug-encapsulating particle core surrounded by a drug-free shell layer,18 often exhibit a reduction in the initial burst release and better controlled release properties19–22 as compared to single-walled ones.
Microorganisms such as yeast can be harnessed as biocompatible and biodegradable reservoirs and have been successfully applied in the encapsulation of essential oil,23 flavor,24,25 antioxidant26,27 and pharmaceuticals.28–30 The cell wall and the plasma membrane of the yeast cell make them an attractive encapsulation matrix.31 Prokaryotes, unicellular cyanobacteria have unique highly differentiated internal membrane systems. Like other Gram-negative bacteria, cyanobacteria such as Synechocystis sp. strain PCC 6803 (PCC) have a cell envelope consisting of a plasma membrane, peptidoglycan layer, and outer membrane,32 thus making them an ideal microencapsulation wall material.
Urea–formaldehyde (UF) resins via in situ polymerization, in which the capsule wall is formed by condensation polymerization on the phase interface, have been employed as the capsule wall, and attracted attention due to its simplicity, low cost and excellent mechanical strength of the resulting capsules.33–36 In situ polymerization has many advantages, such as feasible size controllability and adjustable shell thickness.
Herein, we report a controlled drug-delivery system based on UF modified PCC cells in which TEB are loaded into algae cells. In addition, the uncoated algae cells drug-release system was used for comparison to investigate the release property. Specifically, PCC cells used in this work as appropriate candidates for TEB release system have three distinguishing features: (i) their surface is negatively charged, so they could adsorb and take up positively charged UF prepolymers via electrostatic interactions in acidic solution. (ii) The groups on cell wall are mainly carboxyl, hydroxyl and amine, which are responsible for hydrogen bond formation with TEB. (iii) They are essentially spherical and exhibit narrow size distribution. The uniform property of PCC enables them to be an intelligent drug delivery systems to develop monodispersed microcapsules, which are critical for the precise manipulation of the loading levels and the release kinetics of encapsulated substances.37
Experimental
Materials
Technical grade tebuconazole (98.5% purity) was kindly supplied by Jiannong jiangsu Agrochemical & Chemical Co. Ltd. (China). Tebuconazole (45%, WP, Elite) was obtained from Bayer Co. (Kansas City Mo.). Isopropanol (99.5%), formaldehyde (37%), urea and triethanolamine were obtained from Sinopharm Chemical Regent Co. (China). PCC cells were obtained from the Department of Biology, Shanghai Normal University (China). Double distilled water was used in the experiment. All chemicals were analytic grade and were used without further purification.Fabrication of TEB–PCC
Cyanobacteria used in the experiments were Synechocystis sp. strain PCC 6803 (PCC). The PCC cells were centrifuged, washed with deionized water 3 times, and spray dried at 220 °C. The dried cells (1.0 g), TEB (0.2 g) and absolute ethanol (400 mL) were added into 500 mL capped Erlenmeyer flasks under continuous shaking for 24 h at 30 °C. Then, the cells were centrifuged for 1 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, quickly washed three times with small amounts of absolute ethanol–water mixture (5
000 rpm, quickly washed three times with small amounts of absolute ethanol–water mixture (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) and freeze dried for 48 h. The TEB–PCC was obtained.
95, v/v) and freeze dried for 48 h. The TEB–PCC was obtained.
Preparation of urea–formaldehyde pre-polymer
Recrystallized urea (120 g) and 37% formaldehyde solution (225 mL) were mixed in a 250 mL three-neck round-bottomed flask equipped with a mechanical stirrer at room temperature. When urea was dissolved, the pH value of the resultant solution was adjusted to 8–9 by adding suitable amount of triethanolamine, then the solution was gradually heated to 70 °C and maintained at that temperature for 1 h. At the end of the reaction, this urea–formaldehyde pre-polymer resin was cooled and diluted with distilled water to be 500 mL of UF resin solution. The resin concentration was 0.42 g mL−1.Fabrication of TEB–PCC@UF
TEB–PCC (4 g), prepolymer solution (1 g) and double-distilled water (5.0 mL) were mixed by stirring at 200 rpm at 60 °C, and the pH of the mixture was adjusted to 1.5–2 with 1% HCl solution. The reaction was continued with a stirring rate of 500 rpm for 4 h. After 4 h, the urea–formaldehyde polymer network is formed at the TEB–PCC interface. The resultant microcapsules were filtered and washed with distilled water for three times and dried in a vacuum oven for 20 h.In vitro drug-release study
The different TEB samples containing about 20 mg of TEB were weighted and dispersed in 200 mL of ethanol–water mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) with shaking at 100 rpm, 25 °C, which was used as the release medium in order to dissolve TEB. 2.0 mL of the suspension was removed for analysis at given time intervals with a syringe followed by 10 min centrifugation at 10
50, v/v) with shaking at 100 rpm, 25 °C, which was used as the release medium in order to dissolve TEB. 2.0 mL of the suspension was removed for analysis at given time intervals with a syringe followed by 10 min centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The precipitates from centrifugation and the same volume of fresh release medium were returned to the flasks to keep the composition of suspension unchanged. The extracted medium was sufficiently diluted with release medium, and analyzed by UV/Vis spectroscopy at a wavelength of 220 nm. Blanks containing no TEB and three replicates of each sample were used for each series of experiments.
000 rpm. The precipitates from centrifugation and the same volume of fresh release medium were returned to the flasks to keep the composition of suspension unchanged. The extracted medium was sufficiently diluted with release medium, and analyzed by UV/Vis spectroscopy at a wavelength of 220 nm. Blanks containing no TEB and three replicates of each sample were used for each series of experiments.
Bioassay experiments
The bioactivities of TEB–PCC@UF and commercial formulations of TEB on wheat powdery mildew which is one of main wheat diseases were conducted on 2–3 leaf stage of wheat seedlings. Two formulations were dissolved in water to prepare 100 mg L−1 of the mother liquors and then diluted into 2.5 ppm, 5 ppm, 10 ppm, 20 ppm, 30 ppm and 40 ppm TEB with 0.1% (w/w) Tween 80 solution. The plants were treated homogeneously in a spraying cabin with the pesticides 24 h, 48 h, and 72 h before pathogen inoculation. Wheat was inoculated with pathogens Erysiphe graminis (by shaking spore powder over the uninfected plants), and grown in a greenhouse under conditions of 18 °C, at 70% relative humidity, with 12 h light and were used for testing after 4, 8 and 12 days, respectively.Characterization
The morphology of the samples was characterized by field emission scanning microscopy (FESEM, Hitachi S-4800). Particle size and zeta potential were measured using a Zetasizer Nano-ZS-90 (Malvern Instruments). Fourier transform infrared (FT-IR) spectra were recorded with a Nicolet Magna 550 spectrometer using KBr method. The concentrations of TEB in the adsorption and release experiments were determined by UV-vis spectrophotometer (UV-2000, UNICO (Shanghai) Instruments Co., Ltd.).Results and discussion
Characterization of microcapsules
Scheme 1 schematically illustrates the reduction of initial burst and improvement of efficacy on target fungicide of two drug-delivery systems, TEB–PCC@UF and TEB–PCC. In the former one, TEB release is doubly controlled by the PCC cell shell and the UF layer sequentially. The double-walled TEB–PCC@UF system can exhibit a reduction in the initial burst release and better controlled release properties. For the TEB–PCC, the PCC cell shell can control the drug diffusion with sustained-release behavior only. | ||
| Scheme 1 Schematic illustration of two drug-delivery systems which show different controlled-release patterns. | ||
For the TEB–PCC@UF, the TEB–PCC spheres were effectively covered with UF due to the electrostatic interaction. As shown in Fig. S1,† the zeta potential of TEB–PCC spheres, UF prepolymers, and TEB–PCC@UF were −40.2 mv, 4.32 mv, and −3.98 mv, respectively, indicating that the TEB–PCC spheres have been successfully coated with UF.
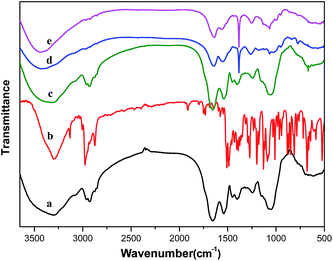
The FT-IR spectrum (Fig. 1) was employed to further prove the presence of UF on TEB–PCC spheres surface. As shown in Fig. 1d and e, the FT-IR spectra of UF resins and TEB–PCC@UF closely match to the characteristic peaks of the N–H stretching vibration at 1543 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1650 cm−1, and a C–H stretching vibration at 1389 cm−1. The peaks at 1242 cm−1 and 3357 cm−1 are assigned to stretching vibrations of C–N bonds, and O–H bonds, respectively. This indicates that the UF resin shell was formed on the surface of TEB–PCC. Furthermore, it is noteworthy to mention that the characteristic adsorption bands of PCC (Fig. 1a) and TEB (Fig. 1b) were not observed at TEB–PCC@UF (Fig. 1e) due to the sealing and penetration resistance of the UF shell resins fully filled with TEB–PCC.33,38,39
O stretching vibration at 1650 cm−1, and a C–H stretching vibration at 1389 cm−1. The peaks at 1242 cm−1 and 3357 cm−1 are assigned to stretching vibrations of C–N bonds, and O–H bonds, respectively. This indicates that the UF resin shell was formed on the surface of TEB–PCC. Furthermore, it is noteworthy to mention that the characteristic adsorption bands of PCC (Fig. 1a) and TEB (Fig. 1b) were not observed at TEB–PCC@UF (Fig. 1e) due to the sealing and penetration resistance of the UF shell resins fully filled with TEB–PCC.33,38,39
The FT-IR spectrum of the TEB–PCC systems was similar to the spectrum of the PCC cells (Fig. 1a and c), whilst the IR absorption bands of TEB significantly decreased, nearly disappeared. This observation suggests that the main bands of TEB was ‘hidden’ by the interaction with the inner wall groups of PCC cell components and TEB molecules are rather located inside the PCC cells. The above results correlate well with Shi and Paramera's26–28 in which the encapsulation of resveratrol, chlorogenic acid and curcumin in yeast cells was studied respectively, and the disappearance of the characteristic IR absorption bands of the substances in the microcapsules was attributed to the encapsulation into cells.
FESEM and dynamic light scattering (DLS) revealed that PCC cells were almost monodisperse microspheres (Fig. 2a) and the average diameter of PCC is relatively uniform (1.474 μm, Fig. S2 in the ESI†). Fig. 2b shows a FESEM micrograph of the PCC after adsorption of TEB molecules (TEB–PCC). No apparent difference can be observed compared to Fig. 2a, although TEB–PCC contains a large number of TEB molecules. Moreover, the adsorption kinetics studies of TEB onto PCC showed that the absorption equilibrium was achieved after 24 h. As obtained from adsorption isotherms (Fig. S3 in the ESI†), the maximum adsorbed amount was about 20.1 mg g−1 at an equilibrium concentration of approximately 500 mg L−1. Consequently, the number of TEB molecules encapsulated in PCC cell sphere is 7.99 × 107 (see the ESI S4†). From the FESEM images (Fig. 2b–d), it clearly depicts that TEB–PCC@UF spheres possess a roughly spherical structure with an average diameter of 1.773 μm, which perfectly replicate the morphology of the cells. Meanwhile, a uniform thin layer with a thickness of around 0.15 μm completely covers the whole outer surface of the TEB–PCC microspheres, which correlate well with the normal FT-IR spectra of TEB–PCC@UF.
To characterize the sustained-release effect, we systematically investigated the release behavior of TEB from the TEB–PCC and TEB–PCC@UF systems in media of ethanol–water mixture (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As shown in Fig. 3a, the amounts of released free TEB reach about 94.6% in less than 53 h (Fig. 3a), and Fig. 3b and c clearly presented that both systems exhibit sustained-release properties. The TEB–PCC system takes about 76 h to release 72% of TEB into the solvent, which might be attributed to the fact that the plasma membrane, peptidoglycan layer, and outer membrane of the PCC cells act as effective barriers preventing the premature release of the TEB from TEB–PCC system.23,24 In addition, it has been reported40–42 that the hydrogen bonds interaction between the carrier and the active ingredient can also affect the release of active ingredient from the matrix. A high energy of interaction would result in a slower release of the active ingredient. Consequently, a large number of hydroxyl and amine groups on the PCC cell (Fig. 1a) act as proton donors for hydrogen bonds, and the nitrogen atoms and hydroxyl groups of TEB act as acceptors. Thus, the hydrogen bond interaction between TEB and the carrier may also contribute to the slow-release of TEB from TEB–PCC system.
1). As shown in Fig. 3a, the amounts of released free TEB reach about 94.6% in less than 53 h (Fig. 3a), and Fig. 3b and c clearly presented that both systems exhibit sustained-release properties. The TEB–PCC system takes about 76 h to release 72% of TEB into the solvent, which might be attributed to the fact that the plasma membrane, peptidoglycan layer, and outer membrane of the PCC cells act as effective barriers preventing the premature release of the TEB from TEB–PCC system.23,24 In addition, it has been reported40–42 that the hydrogen bonds interaction between the carrier and the active ingredient can also affect the release of active ingredient from the matrix. A high energy of interaction would result in a slower release of the active ingredient. Consequently, a large number of hydroxyl and amine groups on the PCC cell (Fig. 1a) act as proton donors for hydrogen bonds, and the nitrogen atoms and hydroxyl groups of TEB act as acceptors. Thus, the hydrogen bond interaction between TEB and the carrier may also contribute to the slow-release of TEB from TEB–PCC system.
For the TEB–PCC@UF system, the TEB release time and rate was obviously decreased compared to TEB–PCC. The release amount only reaches 42% after 76 h. This finding indicates that the UF layer plays an important role and serves as an effective diffusion barrier during the controlled-release process. Furthermore, the t50 values (the time taken for 50% of the TEB to be released, Table 1) were 12.64 h, 34.23 h and 101.62 h for the free TEB, TEB–PCC and TEB–PCC@UF microcapsules, respectively. These results can be concluded that the addition of PCC cells is beneficial to slow down the release of TEB from TEB–PCC microcapsules. Moreover, the TEB–PCC@UF system has a much better controlled drug-release property than the TEB–PCC.
| Sample | Ka (h−1) | nb | R2c | t50d (h) |
|---|---|---|---|---|
| a K is the constant that incorporates the matrix properties.b n is a diffusion parameter.c R is a correlation coefficient.d t50 is the time it takes to release 50% of TEB. | ||||
| TEB–PCC | 14.160 ± 1.984 | 0.387 ± 0.029 | 0.926 | 34.23 |
| TEB–PCC@UF | 3.778 ± 0.661 | 0.536 ± 0.034 | 0.980 | 101.62 |
The major problem in a controlled release system is the obvious initial burst during which a great amount of drug releases, resulting in an acutely high concentration and release lose control.43,44 As shown in Fig. 3, there was an initial burst in the release profile of the free TEB, and it was significantly suppressed in the single-walled TEB–PCC and double-walled TEB–PCC@UF systems. Especially in the double-walled TEB–PCC@UF, its initial burst time is the shortest, suggesting that the double-walled encapsulation of TEB not only can prolong the release time but also depress the initial “burst effect” to some degree.
Drug release studies
Finally, the release mechanisms of TEB were investigated for the TEB–PCC and TEB–PCC@UF microcapsules. The release process should involve two steps: (i) the bulk solution diffuses into the microcapsules and the core TEB dissolves in it. (ii) The dissolved TEB molecules spread out. It is expected that at the early release stage the TEB concentration within the microcapsules is close to saturation, and is sustained until the core TEB dissolve completely.45 During this period, the permeability of the microcapsule shell is the determining factor controlling the release rates, explaining why the release rate decreases as the microcapsule wall thickness increases.46 Here, we analyzed the kinetic release data of the TEB from microcapsules by applying the empirical equation47| Mt/M0 = Ktn | (1) |
Bioassay experiment
To further evaluate the controlled release properties of the TEB–PCC@UF microcapsules, we assessed its protective and persistent effects against wheat powdery mildew. For the test of protective effects (Fig. 4a and b), the plants were inoculated with pathogen of the plant disease for 24 h after spraying with the TEB–PCC@UF microcapsules or the commercial formulation of TEB at doses ranging from 2.5 to 40 mg L−1. It was found that the control efficacies of two formulations were increased with the increasing of TEB concentration sampling at the 4th, 8th and 12th day after TEB application (Fig. 4a and b). However, compared to the commercial formulation, the TEB–PCC@UF microcapsules exhibited excellent control efficacy against wheat powdery mildew, particularly in the concentrations ranging from 10 to 40 mg L−1. This suggested that the TEB–PCC@UF microcapsules can provide significantly protection effect on wheat. For example, at 40 mg L−1 concentration, the control efficacies of TEB–PCC@UF microcapsules at intervals were 95.75%, 88.25% and 83.75%, but that of the commercial product were merely 91.00%, 75.25% and 51.00% respectively. The preferable protective effect can be ascribed to the controlled-release ability of the TEB–PCC@UF microcapsules, which evidently improved the long-term bioavailability of TEB.For the test of persistent effects, the plants inoculated for 24, 48, or 72 h after spray different formulations of TEB at the above concentrations was also studied; and the control efficacies were assessed at the 4th, 8th and 12th day after inoculation, respectively (Fig. 4). TEB–PCC@UF microcapsules also showed a superior persistent effect due to its advantageous controlled-release property. For example, for the 72 h after TEB application, the TEB–PCC@UF microcapsules, especially at high concentrations, exhibited a good control efficacy in 12 days after inoculation. At 40 mg L−1 concentration, the control efficacy still reached 80.75% in 12 days after inoculation. However, the control efficacy of the commercial formulation of TEB only reached 52.25%. Obviously, the TEB–PCC@UF microcapsules had remarkable advantages in controlling TEB release compared to the commercial formulation of TEB.
Conclusions
In summary, we have successfully developed an efficient cyanobacteria cells-based controlled drug-release system by using UF as a coating shell to modify TEB-loaded PCC cells. Compared to the TEB–PCC system without UF, the TEB–PCC@UF system not only can effectively control the drug release rate but also depress the initial “burst effect” to some degree. The UF layer plays an important role and serves as an effective diffusion barrier during the controlled-release process. Furthermore, the control efficacy of TEB–PCC@UF system against wheat powdery mildew remained over 80% in 12 days after inoculation, due to the slow and persistent release of the active components from the system. In contrast, the control efficacy of the commercial formulation at the same concentration (40 mg L−1) only reached 52.25%. Accordingly, the cyanobacteria cells are a promising drug controlled-release platform.Acknowledgements
We thank the National Natural Science Foundation of China (21172147), the National Key Technology R&D Program of China (2011BAE06B06-4), and the National High Technology Research and Development Program of China (863:2011AA100503).References
- X. Q. Huang and M. S. Roder, Euphytica, 2004, 137, 203–223 CrossRef CAS.
- A. K. Sharma, R. K. Sharma and K. S. Babu, Crop Prot., 2004, 23, 249–253 CrossRef PubMed.
- R. N. Strange and P. R. Scott, Annu. Rev. Phytopathol., 2005, 40, 83–116 CrossRef PubMed.
- C. A. Griffey, M. K. Das and E. L. Stromberg, Plant Dis., 1993, 77, 618–622 CrossRef PubMed.
- C. D. S. Tomlin, The e-Pesticide Manual, version 2.2., BCPC, Hampshire, UK, 12th edn, 2002 Search PubMed.
- C. C. Dowler, J. Agric. Food Chem., 1999, 47, 2908–2913 CrossRef CAS PubMed.
- J. Asrar, Y. Ding, R. E. La Monica and L. C. Ness, J. Agric. Food Chem., 2004, 52, 4814–4820 CrossRef CAS PubMed.
- P. Stloukal, P. Kucharczyk, V. Sedlarik, P. Bazant and M. Koutny, J. Agric. Food Chem., 2012, 60, 4111–4119 CrossRef CAS PubMed.
- S. F. Zhang, P. H. Chen, F. Zhang, Y. F. Yang, D. K. Liu and G. Wu, J. Agric. Food Chem., 2013, 61, 12219–12225 CrossRef CAS PubMed.
- R. Grillo, A. E. S. Pereira, N. F. S. de Melo, R. M. Porto, L. O. Feitosa, P. S. Tonello, N. L. D. Filho, A. H. Rosa, R. Lima and L. F. Fraceto, J. Hazard. Mater., 2011, 186, 1645–1651 CrossRef CAS PubMed.
- V. Balmas, G. Delogu, S. Sposito, D. Rau and Q. Migheli, J. Agric. Food Chem., 2006, 54, 480–484 CrossRef CAS PubMed.
- F. Flores-Céspedes, I. Daza-Fernández, M. Villafranca-Sánchez and M. Fernández-Pérez, J. Agric. Food Chem., 2009, 57, 2856–2861 CrossRef PubMed.
- X. J. Wang and J. Zhao, J. Agric. Food Chem., 2013, 61, 3789–3796 CrossRef CAS PubMed.
- D. B. Yang, N. Wang, X. J. Yan, J. Shi, M. Zhang, Z. Y. Wang and H. Z. Yuan, Colloids Surf., B, 2014, 114, 241–246 CrossRef CAS PubMed.
- F. J. Garrido-Herrera, I. Daza-Ferández, E. González-Pradas and M. Fernández-Pérez, J. Hazard. Mater., 2009, 168, 220–225 CrossRef CAS PubMed.
- B. Singh, D. K. Sharma and A. Gupta, J. Hazard. Mater., 2009, 161, 208–216 CrossRef CAS PubMed.
- A. Roy, J. Bajpai and A. K. Bajpai, Carbohydr. Polym., 2009, 76, 222–231 CrossRef CAS PubMed.
- K. J. Pekarek, J. S. Jacob and E. Mathiowitz, Nature, 1994, 367, 256–260 CrossRef PubMed.
- Y. J. Xia, Q. X. Xu, C. H. Wang and D. W. Pack, J. Pharm. Sci., 2013, 102, 1601–1609 CrossRef CAS PubMed.
- Y. J. Xia, P. F. Ribeiro and D. W. Pack, J. Controlled Release, 2013, 172, 707–714 CrossRef CAS PubMed.
- H. X. Tan and J. D. Ye, Appl. Surf. Sci., 2008, 255, 353–356 CrossRef CAS PubMed.
- S. He, W. B. Zhang, D. G. Li, P. L. Li, Y. C. Zhu, M. M. Ao, J. Q. Li and Y. S. Cao, J. Mater. Chem. B, 2013, 1, 1270–1278 RSC.
- J. R. P. Bishop, G. Nelson and J. Lamb, J. Microencapsulation, 1998, 15, 761–773 CrossRef CAS PubMed.
- V. Normand, G. Dardelle, P. E. Bouquerand, L. Nicolas and D. Johnston, J. Agric. Food Chem., 2005, 53, 7532–7543 CrossRef CAS PubMed.
- G. Dardelle, V. Normand, M. Steenhoudt, P. E. Bouquerand, M. Chevalier and P. Baumgartner, Food Hydrocolloids, 2007, 21, 953–960 CrossRef CAS PubMed.
- G. R. Shi, L. Q. Rao, H. Z. Yu, H. Xiang, G. P. Pen, S. Long and C. Yang, J. Food Eng., 2007, 80, 1060–1067 CrossRef CAS PubMed.
- G. R. Shi, L. Q. Rao, H. Z. Yu, H. Xiang, H. Yang and R. Ji, Int. J. Pharm., 2008, 349, 83–93 CrossRef CAS PubMed.
- E. I. Paramera, S. J. Konteles and V. T. Karathanos, Food Chem., 2011, 125, 892–902 CrossRef CAS PubMed.
- S. Blanquet, G. Garrait, E. Beyssac, C. Perrier, S. Denis, G. Hebrard and M. Alric, Eur. J. Pharm. Biopharm., 2005, 61, 32–39 CrossRef CAS PubMed.
- S. Blanquet, S. Marol-Bonnin, E. Beyssac, D. Pompon, M. Renaud and M. Alric, Trends Biotechnol., 2001, 19, 393–400 CrossRef CAS.
- P. N. Lipke and R. Ovalle, J. Bacteriol., 1998, 180, 3735–3740 CAS.
- E. Hoiczyk and A. Hansel, J. Bacteriol., 2000, 182, 1191–1199 CrossRef CAS.
- R. Qin, G. Y. Xu, L. Guo, Y. Jiang and R. Y. Ding, Mater. Chem. Phys., 2012, 136, 737–743 CrossRef CAS PubMed.
- J. P. Wang, X. P. Zhao, H. L. Guo and Q. Zheng, Langmuir, 2004, 20, 10845–10850 CrossRef CAS PubMed.
- S. Cosco, V. Ambrogi, P. Musto and C. Carfagna, J. Appl. Polym. Sci., 2007, 105, 1400–1411 CrossRef CAS.
- L. Yuan, F. Chen, A. J. Gu, G. Z. Liang, C. Lin, S. D. Huang, S. Nutt, G. Q. Chen and Y. M. Gao, Polym. Bull., 2014, 71, 261–273 CrossRef CAS PubMed.
- L. Y. Chu, A. S. Utada, R. K. Shah, J. W. Kim and D. A. Weitz, Angew. Chem., Int. Ed., 2007, 46, 8970–8974 CrossRef CAS PubMed.
- S. J. Park, Y. S. Shin and J. R. Lee, J. Colloid Interface Sci., 2001, 241, 502–508 CrossRef CAS.
- H. L. Guo and X. P. Zhao, Opt. Mater., 2004, 26, 297–300 CrossRef CAS PubMed.
- J. V. Cottefill, R. M. Wilkins and F. T. da Silva, J. Controlled Release, 1996, 40, 133–142 CrossRef.
- Y. Yi, S. Xu, H. K. Sun, D. Chang, Y. H. Yin, H. Zheng, H. X. Xu and Y. C. Lou, Carbohydr. Polym., 2011, 86, 1007–1013 CrossRef CAS PubMed.
- J. Li, Y. Li and H. Dong, J. Agric. Food Chem., 2008, 56, 1336–1342 CrossRef CAS PubMed.
- C. D. Herzfeldt and R. Kuemmel, Drug Dev. Ind. Pharm., 1983, 9, 767–793 CrossRef CAS.
- L. Vayssieres, C. Chaneac, E. Tronc and J. P. Jolivet, J. Colloid Interface Sci., 1998, 205, 205–212 CrossRef CAS PubMed.
- A. A. Antipov, G. B. Sukhorukov, E. Donath and H. Moehwald, J. Phys. Chem. B, 2001, 105, 2281–2284 CrossRef CAS.
- X. Qiu, S. Leporatti, E. Donath and H. Moehwald, Langmuir, 2001, 17, 5375–5380 CrossRef CAS.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 23–36 CrossRef CAS.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 37–42 CrossRef CAS.
- S. Conti, L. Maggi, L. Segale, E. Ochoa Machiste, U. Conte, P. Grenier and G. Vergnault, Int. J. Pharm., 2007, 333, 143–151 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01629k |
| This journal is © The Royal Society of Chemistry 2015 |