Modulation of human mesenchymal stem cell survival on electrospun mesh with co-immobilized epithelial growth factor and gelatin
Abstract
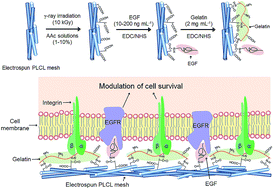
In this work, we present a biomimetic fibrous scaffold containing two biomolecules. A biocompatible poly(L-lactide-co-ε-caprolactone) mesh was fabricated by an electrospinning method, and then acrylic acid was grafted on the mesh to introduce a carboxyl group through γ-ray irradiation. Subsequently, the epidermal growth factor (EGF) and gelatin were coupled to the mesh through the EDC reaction. The modified mesh presents a consistent fibre diameter (874.4 ± 178.5 nm), with carboxyl groups (1.3 mM). EGF (171.7 ng mg−1 mesh) and gelatin (67.2 ± 30.5 μg mg−1 mesh) were successfully coupled on the mesh. The coupled EGF and gelatin promoted the cell viability 1.5-times higher than that from a non-modified mesh. In particular, the EGF on the meshes independently allowed hMSC to present a 3-times greater involucrin expression and enabled improved procollagen secretion, implying trans-differentiation of hMSC to keratinocyte-like cells. Therefore, the co-immobilization strategy of biomolecules using radiation technology may be an alternative tool for tissue engineering applications.

 Please wait while we load your content...
Please wait while we load your content...