Intense UV upconversion through highly sensitized NaRF4:Tm (R:Y,Yb) crystals†
Abstract
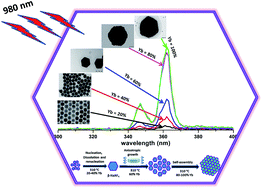
Photon upconverting luminescent hexagonal NaRF4:Tm (0.5 mol%) (R:Y3+,Yb3+) crystals with Yb3+ concentrations between 20 and 99.5 mol% were synthesized by a modified thermal coprecipitation method. The effects of the Yb3+ sensitizer concentration on the shape, size, structure, upconversion luminescence intensities and dynamic luminescence lifetimes were studied in detail. The intensity of ultraviolet upconversion luminescence at 340–365 nm upon 980 nm excitation increased up to 20 times with increasing Yb3+ concentration. The mechanisms for the changes in morphology, size and UV upconversion emission are discussed. In addition, the effect of the Tm3+ concentration on the UV emission of NaYbF4 crystals was investigated. The results demonstrate that the NaYbF4 with Tm3+ concentration between 0.4 to 0.8 mol% produce the most intense UV upconversion luminescence. These crystals may find applications in e.g. UV-visible solid state lasers or as an internal UV radiation source for many photochemical reactions.

 Please wait while we load your content...
Please wait while we load your content...