Bioinspired nanostructured hydroxyapatite/collagen three-dimensional porous scaffolds for bone tissue engineering
Junjie Guan†
a,
Jun Yang†b,
Junqi Daia,
Yunhao Qina,
Yang Wanga,
Yaping Guo*b,
Qinfei Ke*b and
Changqing Zhang*a
aDepartment of Orthopedics Surgery, Shanghai Jiaotong University Affiliated Sixth People's Hospital, Shanghai 200233, China. E-mail: zhangcq@sjtu.edu.cn; Fax: +86-21-64363802; Tel: +86-21-24058009
bThe Education Ministry Key Lab of Resource Chemistry, Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Normal University, Shanghai 200234, P. R. China. E-mail: kqf@shnu.edu.cn; ypguo@shnu.edu.cn; Fax: +86-21-64321951; Tel: +86-21-64321951
First published on 14th April 2015
Abstract
During the biomineralization process of bone minerals, amorphous calcium phosphate (ACP) is converted to apatite crystals by using octacalcium phosphate (OCP) and brushite (DCPD) as transitory precursors, resulting in the formation of hybrid nanostructured collagen/apatite composites. Herein, we report, for the first time, the bioinspired synthesis of a collagen/hydroxyapatite (HA) porous scaffold (CHPS) according to the following stages: (i) fabrication of collagen fibre porous scaffold (CFPS) by a needle-punching process; (ii) deposition of brushite/chitosan (DCPD/CS) on CHPS by a dip-coating method; and (iii) formation of CHPS by in situ conversion of DCPD to HA. The CHPS exhibits three-dimensional (3D) interconnected porous structures with pore sizes of around 60 μm. HA crystals distribute homogeneously on the CHPS, and display wheat-like shapes with a length of approximately 200 nm and a width of approximately 80 nm. The in vitro cell tests by using human bone marrow stromal cells (hBMSCs) indicate that the HA crystals in the CHPS not only promote the cell adhesion and proliferation of the hBMSCs, but also stimulate osteogenic differentiation. The in vivo results reveal that the CHPS exhibits better osteoinductivity than the CFPS because of its similar chemical components, crystallinity and crystallographic texture to natural bone. Moreover, the CHPS can stimulate new bone formation in rat critical-sized calvarial defects within 8 weeks. The CHPS possesses a favourable pore structure, and excellent biocompatibility and osteoinductivity, and thus it has great potential applications for bone tissue engineering.
1 Introduction
Bone tissue engineering is a rapidly developing discipline to repair, replace or regenerate injured bone tissue. Investigation and simulation of the chemical components and hierarchical microstructures of natural bone offer a favour strategy for the synthesis of artificial bone scaffolds. Natural bone is a highly complex and well-organized tissue, which is composed of around 25 wt% type I collagen nanofibrils, 65 wt% apatite crystals and 10 wt% water.1,2 Collagen fibrils form a scaffold for a highly organized arrangement of uniaxially oriented apatite crystals. However, the synthesis of artificial scaffold similar to natural bone in chemical components, crystallographic texture, crystallinity and porous structure still remains a big challenge.Apatite is a major inorganic component of natural bone, and the corresponding synthetic hydroxyapatite (HA) has been widely used as an artificial bone scaffold due to its good biocompatibility, bioactivity and osteoinductivity. Notably, the biological properties of HA depend mainly on the morphology, crystallinity and crystal orientation. The thickness of biological apatite crystals is 3–10 nm, and the width (or length) is 30–200 nm.2 The HA crystals in bone minerals exhibit poor crystallinity due to the incorporation of impurities such as carbonate, sodium and magnesium ions.2 The HA crystals with low crystallinity possess better bioactivity and biodegradability than those with high crystallinity.3 In vertebrate bones and tooth enamel surfaces, HA crystals exhibit c-axis orientation and a(b)-axis orientation, respectively. Kim et al. have found that c-axis oriented HA coatings have higher hardness and Young's modulus values compared with the values of randomly oriented coatings.4 In simulated physiological solution, the c-axis oriented HA surfaces can promote preferentially oriented growth through a cyclic process of dissolution and reprecipitation, followed by homoepitaxial growth. In vivo tests shows that ordered rod-like fluorapatite coatings can accelerate and enhance mineralized tissue formation.5 Moreover, the HA crystals with the surfaces exhibiting a tailored crystallographic texture enable a new level of control of cellular behaviour and enhance mineralized tissue formation.4–6
The biological properties of bone scaffolds are associated not only with their chemical composition, crystallinity and crystal orientation, but also with their porous structure.7,8 The interconnected pores allow cells to penetrate and grow in the scaffolds, and facilitate the transport of nutrients, oxygen and growth factors.9 In recent years, several fabrication techniques have been developed to establish porous scaffolds, which include thermally induced phase separation, solid-free fabrication, and particulate leaching.10–12 However, the scaffolds that are prepared by the above methods exhibit usually poor mechanical properties.
In bone tissue, collagen fibrils form a scaffold for a highly organized arrangement of uniaxially oriented apatite crystals. The apatite is converted from amorphous calcium phosphate (ACP) by using metastable crystalline phases such as octacalcium phosphate (OCP, Ca8H2(PO4)6·5H2O) and brushite (DCPD, CaHPO4·2H2O) as transitory precursors.2 Herein, we fabricated collagen/HA porous scaffold (CHPS) according to the following steps: firstly, collagen fibre porous scaffold (CFPS) was fabricated by a needle-punching process; secondly, collagen/DCPD porous scaffold (CDPS) was formed by deposition of chitosan (CS)/DCPD on CFPS; thirdly, CHPS was converted from CDPS after treatment with a NaOH solution. The main aims of the present work were to fabricate CHPS, to investigate its cytocompatibility by using human bone marrow stromal cells (hBMSCs) as cell model, and to discuss its osteoinductivity and bioactivity by using a rat calvaria defect model.
2 Experimental
2.1 Preparation of CFPS
The CFPS was produced with the needle punching process using a needle machine (F22G-I1600, ChangshuWeicheng Non-Woven Equipment Co., Ltd, Jiangsu, China). The thickness and surface density of the fiber scaffold were 4.74 ± 0.35 mm and 444.12 ± 31.57 g m−2, respectively.2.2 Preparation of CHPS
One gram of chitosan powder was dissolved in 100 mL of acetic acid solution (2 wt %) under vigorous agitation to obtain a homogeneous solution. Ca(NO3)2·4H2O (2.3615 g) and 0.9361 g of NaH2PO4·2H2O were added to the chitosan/acetic solution at room temperature. The CFPS was immersed in the above mixed solution for 5 min and was then withdrawn at a rate of 1 mm s−1. After drying at 40 °C for 48 h, the samples were immersed in NaOH solution (5 wt %) at 37 °C for different time intervals. Finally, the obtained CHPS was dried at 40 °C for 1 day in a humid atmosphere.2.3 Characterization
The morphology and microstructure of the samples were investigated with scanning electron microscopy (SEM, MX2600, CamScan). Attenuated total reflectance (ATR) Fourier transform infrared (FTIR) spectra were obtained using a FTIR-Raman (Nexus 670, Thermo Fisher Nicolet Co., Massachusetts, USA). The phases of samples were examined by X-ray power diffraction (XRD, D/max-II B, Japan). The thermal behaviours of samples were examined by thermo-gravimetric analysis (TG-DTA, Perkin-Elmer) at a heating rate of 10 °C min−1 in an alumina crucible in air atmosphere. The compressive strength of the CHPS (25 × 25 × 30 mm) was tested using a universal material testing machine 2T (WDW3020, Changchun New Test Instrument Co., Ltd., China) with a compression speed of 0.5 mm min−1. The flexure strength of CHPS (50 × 20 × 3 mm) was tested using a microcomputer control electronic universal testing machine (WDW-20, Shanghai Hualong Microelectronics Co., Ltd., China) with a chuck travelling speed of 1 mm min−1. The tensile strength of CHPS (50 × 20 × 3 mm) was tested using an electrical universal material testing machine (YG028-500, Changzhou First Textile Machinery Co., Ltd., China) with a stretching velocity of 10 mm min−1 and a gauge length of 40 mm. The pore structure of samples was measured with a Capillary Flow Porometer (CFP, Through-pore size analyzer, Porometer 3G zh, Quantachrome Instruments Ltd., Florida, USA).| P = (W1 − W2)/(ρV) | (1) |
2.4 Preparation of CFPS and CHPS and in vitro cell seeding
All experiments that required the handling of human tissue were approved by the Research Ethical committee of the Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Fresh human bone marrow was obtained from three patients who had received surgery at the Department of Orthopedics of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. All patients provided informed consent. The cell isolation and culture were performed as previously described.13,14 Cells from passage 5 were collected by treatment with trypsin/EDTA solution for all experiments.Prior to cell seeding, scaffolds with a diameter of 15 mm and a thickness of 3 mm were rinsed in 75% ethanol for 2 h. Then, the scaffolds were extensively washed with PBS to remove the residual ethanol. Twenty thousand cells were seeded onto the scaffolds. The cells were allowed to adhere for 3 h. Subsequently, α-MEM with 10% FBS was added. Twenty thousand cells were seeded onto the polystyrene plates as control.
2.5 Observation of cell morphology
The cell morphology was observed via scanning electron microscopy (SEM) (FESEM, Hitachi S-4800, CamScan). After three days in culture, the specimens were fixed with 2.5% glutaraldehyde (pH 7.4) for 24 h. After washing 3 times with PBS, the specimens were dehydrated in graded concentrations of ethanol. The constructs were then critical-point dried. Each sample was coated with gold prior to examination.2.6 Cell viability
To examine the cell viability within the scaffolds, the constructs were stained using the ScienCell™ live/dead assay kit. Briefly, the scaffolds (days 7) were incubated with working staining solution. The constructs were observed immediately under confocal laser microscopy (Carl Zeiss). Live cells stained fluorescently green, while dead cells appeared red.2.7 Cell proliferation assay
The cell proliferation was analyzed using CCK-8 assay kit as previously described.15 Briefly, cells were cultured at 37 °C in a 5% CO2 incubator. After 1, 3, 5, 7, 10 and 14 days, 100 μl CCK-8 solution was added to each well. After another 3 h of culture in an incubator, the absorbance of each well was measured with a microplate reader (BioTek) and a 450 nm filter. All experiments were performed three times.2.8 ALP activity
After 7 and 14 days cultured with α-MEM with 10% FBS, the cells were collected and the ALP activity was measured to assess the osteogenic differentiation. Briefly, the scaffolds were thoroughly washed with PBS to discard the residual serum. The samples were then rinsed in 0.02% Triton X-100 to dissolve the cells. The solution was collected and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 15 min. The supernatant was transferred into 1.5 ml tubes that contained 100 μl of 1 M Tris-HCL, 20 μl 5 mm MgCl2, and 20 μl 5 mM p-nitrophenyl phosphate was added. After incubation at 37 °C for 30 min, the reaction was stopped by the addition of 50 μl of 1 N NaOH. The absorbance at 405 nm was measured, and the ALP activities were normalized to the total protein content determined using the BCA assay kit as previously described.16
000 rpm and 4 °C for 15 min. The supernatant was transferred into 1.5 ml tubes that contained 100 μl of 1 M Tris-HCL, 20 μl 5 mm MgCl2, and 20 μl 5 mM p-nitrophenyl phosphate was added. After incubation at 37 °C for 30 min, the reaction was stopped by the addition of 50 μl of 1 N NaOH. The absorbance at 405 nm was measured, and the ALP activities were normalized to the total protein content determined using the BCA assay kit as previously described.16
2.9 Calcium contents
The cell-scaffold composition was detected for calcium deposition using a calcium assay kit (Sigma). Briefly, the constructs were homogenized in 0.5 N HCl solution for 24 h. The working solution was added to each sample following the manufacturer's instruction. The absorbance was measured at 560 nm.2.10 In vivo implantation
The animal experiments were approved by the Animal Experiment Committee at Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Twenty SD rats (250–300 g) were randomly divided into two groups: the CFPS and CHPS group. Five rats were used as untreated controls. The surgical procedures were performed as previously described.17,18 Briefly, a 15 mm linear incision was made on the skull, and the bone was revealed by blunt dissection. A 6 mm full-thickness bone defect was created on both sides of the skull with a slow-speed dental drill. The site was irrigated with 0.9% physiological saline for cooling. The defects were randomly implanted with CFPS or CHPS or left empty. The incisions were closed, and the animals were allowed access to food and activity ad libitum.2.11 Sequential fluorescent labeling
To label the mineralizing tissues, the animals were intraperitoneally injected with calcein (10 mg kg−1; Sigma) and alizarin red (20 mg kg−1; Sigma) 4 and 6 weeks after the surgery, as previously described.192.12 Micro-CT tomography
The rats were sacrificed 8 weeks after the surgery. The bone volume in the defect site was assessed using a micro-CT imaging system (Skyscan, 1076 scanner, Kontich, Belgium) as previously described.20 Briefly, the specimens were scanned through a 180° rotation angle with a rotation step of 0.6° at a resolution of 18 μm. The digital image was reconstructed using the NRecon Software to visualize the 3D representation of the defect site. The extent of bone regeneration within the calvaria defects was calculated using the CT analysis software. All reconstructions and analyses were performed using the same standardized threshold.2.13 Fluorescent and histological analysis
The skull was fixed in 10% buffered formalin (pH 7.4). Some samples were dehydrated in increasing concentrations of alcohol and then embedded in polymethylmethacrylate (PMMA). For further analysis, the specimens were cut into 250 μm thick sections using a saw microtome (Leica, Germany) and ground and polished to a final thickness of 40 μm. The fluorescent labeling of sections was observed under a confocal laser scanning microscope (CLSM) (Carl, Zeiss). The following excitation/emission wavelengths were used: 543/580–670 nm (alizarin red, red) and 488/500–550 nm (calcein, green). For histological analysis, the sections were stained with van Gieson's picrofuchsin and measured the percent of newly formed bone as previously described.212.14 Statistical analysis
The experimental results are presented as the mean ± SD. The statistical significance was assessed by ANOVA, where p < 0.05 was considered statistically significant. All in vitro results are representative of at least three independent experiments.3 Results
3.1 Characteristics of CHPS
After soaking in a NaOH solution for 1 day, CDPS is converted to CHPS. The SEM image in Fig. 3a indicates that the CHPS possesses 3D interconnected macroporous structure, which is similar to CFPS and CDPS. The pore size distribution curves demonstrate that the pore sizes of CFPS and CHPS are mainly distributed around 80 and 60 μm, respectively. The smaller pore size of the CHPS than the CFPS is attributed to the presence of HA crystals on the surfaces of collagen fibres (Fig. 3b). The high-resolution SEM image shows the HA rods with a length of ∼200 nm and width of ∼80 nm are perpendicularly oriented to the collagen fibres. Interestingly, these HA rods possess the hierarchical nanostructure, which are composed of many smaller nanorods oriented along the c-axis orientation. The length and width of the nanorods is approximately 50 nm and 10 nm, respectively. The corresponding ED pattern shows the visible diffraction rings, which are indexed according to the crystalline HA phase (Fig. 3f). The EDS spectrum in Fig. 3d reveals that the chemical elements of the CHPS mainly include Ca, P, O, C and Na. Ca, P, O are derived from HA, C is derived from collagen and CS, and Na is mainly due to the adsorption of Na+ ions on the scaffold or the substitution of the Ca2+ ions in HA crystal lattice by Na+ ions.
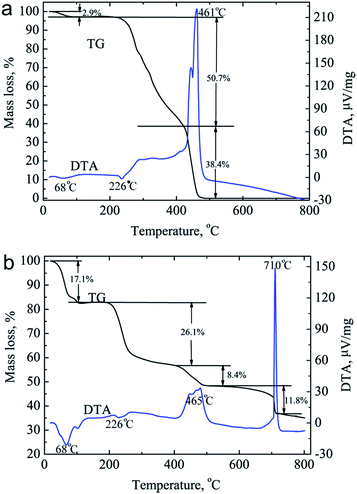
The functional groups of the coatings on the DCPD and CHPS are demonstrated by FTIR spectrum in Fig. 4b. For the CDPS, the infrared stretching v(OH) are observed at 3550, 3489 and 3412 cm−1. The band at 1648 cm−1 is due to the H–O–H bending of lattice water molecules.22 The bands at 1147, 1070, 986 and 877 cm−1 are ascribed to P–O stretching vibration in HPO42− groups, respectively.22 The band due to O–P–O(H) bending mode locates at 530 cm−1. For the CHPS, the intense absorption peak at 1030 cm−1 is ascribed to the stretching vibration (v3) of the phosphate (PO43−) groups, and the absorption peaks at 563 and 604 cm−1 are ascribed to the bending vibration (v4) of the phosphate (PO43−) groups.23 The absorption band due to HPO42− at around 1102 cm−1 indicates that the samples are calcium-deficient HA.24 The band at 3437 cm−1 is corresponding to OH group in HA crystals or adsorbed water on the composite scaffold.25 The characteristic bands of CS at 1420 cm−1 are observed in the CDPS and CHPS (Fig. 4b). However, its characteristic bands at 1080 and 3437 cm−1 are overlapped by those of HA crystals (Fig. 4b).
| Samples | Compression strength | Flexure strength | Tensile strength |
|---|---|---|---|
| CFPS | 1.32 ± 0.30 MPa | ||
| CHPS | 6.12 ± 0.35 MPa | 3.71 ± 0.18 MPa | 3.75 ± 0.61 MPa |
| Trabecular bone26 | 4–12 Mpa |
3.2 Cell performances of hBMSCs on CHPS
The scaffolds are stained for CCK-8 1, 3, 5, 7, 10 and 14 days after seeding to reveal the proliferation of hBMSCs. As shown in Fig. 6e, the number of viable cells on both the CFPS and CHPS continue to increase for 10 days. Cells on both the plate and scaffolds display a lag in cellular proliferation till 14 days of culture. Compared with the plate control or CFPS, the hBMSCs cultured on CHPS exhibit significantly enhanced metabolic activity at each time-point, suggesting that the CHPS promoted the proliferation of hBMSCs. The CCK-8 results show that the addition of HA crystals significantly increased cell proliferation.
3.3 Biocompatibility and osteogenesis in vivo
4 Discussion
In bone tissue engineering, scaffolds are fabricated to provide a temporary, artificial extracellular matrix to support cell attachment, encourage osteo-induction and enhance osteo-integration with the host bone.27 One strategy to fabricate such a scaffold is to design architecture and components that are similar to skeletal bone.28 To mimic the components and architecture of human bone, we fabricate CHPS according to the following steps (Fig. 11): (i) fabrication of CFPS by needle-punching process (Fig. 11a and b); (ii) preparation of CDPS by deposition of CS/DCPD on CFPS (Fig. 11c and d); and (iii) conversion of CHPS from CDPS by treatment with a NaOH solution (Fig. 11e). HA crystals on the CHPS show an ear of wheat-like morphology with a length of approximately 200 nm and width of 80 nm (Fig. 3c). The pore size of the CHPS is 60 μm, which is slightly smaller than that of the CFPS. | ||
| Fig. 11 Fabrication of the CHPS: (a) CO fibers; (b) construction of CFPS by needle-punching; (c) dip-coating process; (d) drying at 37 °C; (e) alkaline solution treatment. | ||
The porous scaffold is believed to mimic the in vivo microenvironment, which provides a favorable template for tissue regeneration. In addition, the large surface area and volume of the porous scaffold allows the transport of nutrients and metabolites. Various techniques to fabricate porous scaffold have been reported, including phase separation,29 supercritical foaming30 and 3D printing.31 However, these porous scaffolds cannot solve the problems of pore size, interconnectivity or cytotoxicity.32 The current study utilizes the needle-punching technique to build the porous scaffold. The architectural parameters of CHPS (e.g., porosity, pore size, and interconnectivity) can be precisely controlled with the needle-punching technique. The CHPS exhibits three-dimensional (3D) interconnected porous structures with pore sizes of around 60 μm. In our previous study, we used the needle punching technique to fabricate porous scaffold,33,34 and these scaffolds were highly porous and cytocompatible. Notably, the processing routes involve only a physical change and did not utilize or generate toxic chemicals.
The biological properties of HA, such as osteoconductivity and biocompatibiltiy, are directly related to its morphology, crystallinity and crystal orientation.35,36 Therefore, extensive efforts have been made to develop HA crystal with similar geometry, crystallinity and crystal orientation in natural bone. Several techniques for fabricating nanoscale HA have been developed in the past few decades, including wet chemical precipitation,37 solvent-cast38 and mechanochemical synthesis.39 However, fabricating biological apatite crystals is still a challenge. In this study, we use bioinspired mineralization to form biological HA with low crystallinity and c-axis orientation. The high-resolution SEM image shows the HA rods possess the hierarchical nanostructure, which are composed of many smaller nanorods oriented along the c-axis orientation. The length and width of the nanorods is approximately 50 nm and 10 nm, respectively. The HA crystals play an important role in determining the surface topography of the CHPS, which significantly enhance the proliferation and differentiation of hBMSCs (Fig. 6 and 7).
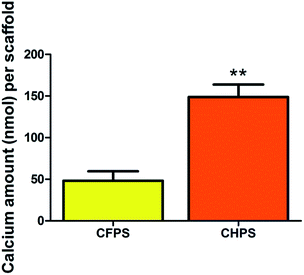
hBMSCs are promising and attractive cell source for bone tissue regeneration.40 Numerous studies have utilized hBMSCs and porous scaffold to repair bone defects.41 Therefore, we use hBMSCs as a cell model to investigate the biocompatibility and osteoinductivity of the CHPS. The proliferation of hBMSCs on the CHPS is qualitatively determined with CCK-8 assay. According to the results, the CHPS significantly promote cell proliferation. An ideal bone scaffold not only can enhance hBMSCs proliferation, but also possess the ability to stimulate the osteogenic differentiation of hBMSCs. ALP is a marker of bone differentiation and mineralization. We quantitatively evaluate the osteogenic differentiation of hBMSCs using the ALP activity. After 7 and 14 days in culture, the hBMSCs in the CHPS exhibit higher ALP activity than those in the CFPS, demonstrating that the CHPS significantly affect the osteoblastic differentiation of hBMSCs. The calcium content also shows that hBMSCs on CHPS yield more calcium composition in comparison with CFPS. According to the in vitro results, the CHPS are highly biocompatible and osteoinductive.
CFPS or CHPS is implanted in rat calvarial defect to repair bone defect. All rats remain in good health and do not show any wound complications, which reveal the excellent biocompatibility of the two scaffolds. The bone healing process is monitored by radiographical and histological studying of the specimens. The results reveal that CHPS significantly enhance new bone formation. However, only little new bone formation is observe in the blank control and CFPS groups. Further analysis of micro-CT indicates that both BV/TV and BMD in the CHPS groups is significant higher than that in the CFPS and blank control (Fig. 8). The histological staining shows that newly formed bone completely bridge the defect area in CHPS groups, whereas the newly formed bone only partly cover the defect area in the CFPS groups. The quantification of new bone area in the CHPS groups is much higher than in the CFPS, which further demonstrate the above results (Fig. 10). The in vivo results show that CHPS possess better osteoinductivity, which may due to its promoting stem cells proliferation and osteogenic differentiation. Besides, CHPS possess similar chemical component, crystallinity and crystallographic texture to natural bone, resulting in excellent integration with surrounding tissues. In conclusion, the in vivo results suggest that CHPS can effectively enhance new bone formation in vivo.
A suitable degradation rate is an essential requirement of scaffolds. The in vivo degradation of CFPS and CHPS was observed after 8 weeks implantation. The CHPS displayed remarkable degradation, accompanied by more newly formed bone in comparison with CFPS. The reason for different degradation behavior of CFPS and CHPS may associate with the osteoclast activity, mechanical strength and local microenvironment. However, the degradation behavior of CFPS and CHPS requires large animal experiment and future study.
5. Conclusions
The highly bioactive and biocompatible CHPS is designed and fabricated by needle punching and bioinspired mineralization technique. The physical architecture and chemical composition of the CHPS is similar to those of human bone. The CHPS is highly porous and interconnected with a pore size of 60 μm. HA crystals distribute homogeneously on CHPS, and display wheat-like shapes with a length of approximately 200 nm and width of approximately 50 nm. The in vitro experiments show that the CHPS enhance the attachment, proliferation, and osteogenic differentiation of hBMSCs. Furthermore, the in vivo experiment indicate that CHPS significantly enhance new bone formation in rat calvarial defects as compare with the CFPS and blank control groups. Due to its similar chemical component, crystallinity and crystallographic texture to natural bone, CHPS represents a promising scaffold for bone tissue engineering.Acknowledgements
This research was supported by Natural Science Foundation of China (Nos. 51002095 and 51372152), Science and Technology Commission of Shanghai Municipality (no. 12JC1405600), Program of Shanghai Normal University (no. DZL124, DCL201303), Innovation Foundation of Shanghai Education Committee (no. 14ZZ124), National High Technology Research and Development Program of China (2012AA020506), “Priority Among Priorities” Clinical Medical Center Construction Project of the Shanghai Municipal, Doctoral Innovation Fund of Shanghai Jiao Tong University School of Medicine (BXJ201341), and State Key Laboratory for Modification of Chemical Fibres and Polymer Materials, Dong Hua University.Notes and references
- F. Nudelman, K. Pieterse, A. George, P. H. H. Bomans, H. Friedrich, L. J. Brylka, P. A. J. Hiblers, G. de With and N. A. J. M. Sommerdijk, Nat. Mater., 2010, 9, 1004–1009 CrossRef CAS PubMed.
- M. J. Olszta, X. Cheng, S. S. Jee, R. Kumar, Y.-Y. Kim, M. J. Kaufma, E. P. Douglas and L. B. Gower, Mater. Sci. Eng., R, 2007, 58, 77–116 CrossRef PubMed.
- S. Rakmae, Y. Ruksakulpiwat, W. Sutapun and N. Suppakarn, Mater. Sci. Eng., C, 2012, 32, 1428–1436 CrossRef CAS PubMed.
- H. Kim, R. P. Camata, S. Chowdhury and Y. K. Vohra, Acta Biomater., 2010, 6, 3234–3241 CrossRef CAS PubMed.
- J. Liu, X. Wang, Q. Jin, T. Jin, S. Chang, Z. Zhang, A. Czajka-Jakubowska, W. V. Giannobile, J. E. Nör and B. H. Clarkson, Biomaterials, 2012, 33, 5036–5046 CrossRef CAS PubMed.
- Z. Zhuang, T. J. Fujimi, M. Nakamura, T. Konishi, H. Yoshimura and M. Aizawa, Acta Biomater., 2013, 9, 6732–6740 CrossRef CAS PubMed.
- C. M. Murphy, M. G. Haugh and F. J. O'Brien, Biomaterials, 2010, 31, 461–466 CrossRef CAS PubMed.
- M. C. Phipps, W. C. Clem, J. M. Grunda, G. A. Clines and S. L. Bellis, Biomaterials, 2012, 33, 524–534 CrossRef CAS PubMed.
- S. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30, 546–554 CrossRef CAS PubMed.
- Y. S. Nam and T. G. Park, J. Biomed. Mater. Res., 1999, 47, 8–17 CrossRef CAS.
- J. M. Taboas, R. D. Maddox, P. H. Krebsbach and S. J. Hollister, Biomaterials, 2003, 24, 181–194 CrossRef CAS.
- V. P. Shastri, I. Martin and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 1970–1975 CrossRef CAS.
- B. Rai, J. L. Lin, Z. X. H. Lim, R. E. Guldberg, D. W. Hutmacher and S. M. Cool, Biomaterials, 2010, 31, 7960–7970 CrossRef CAS PubMed.
- A. K. Gaharwar, S. Mukundan, E. Karaca, A. Dolatshahi-Pirouz, A. Patel, K. Rangarajan, S. M. Mihaila, G. Iviglia, H. Zhang and A. Khade-mhosseini, Tissue Eng., Part A, 2014, 20, 2088–2101 CrossRef CAS PubMed.
- Z. Yin, X. Chen, J. L. Chen, W. L. Shen, T. M. Hieu Nguyen, L. Gao and H. W. Ouyang, Biomaterials, 2010, 31, 2163–2175 CrossRef CAS PubMed.
- J. Zhang, H. Zhou, K. Yang, Y. Yuan and C. Liu, Biomaterials, 2013, 34, 9381–9392 CrossRef CAS PubMed.
- C. M. Cowan, Y. Y. Shi, O. O. Aalami, Y. F. Chou, C. Mari, R. Thomas, N. Quarto, C. H. Contag, B. Wu and M. T. Longaker, Nat. Biotechnol., 2004, 22, 560–567 CrossRef CAS PubMed.
- J.-H. Ye, Y.-J. Xu, J. Gao, S.-G. Yan, J. Zhao, Q. Tu, J. Zhang, X.-J. Duan, C. A. Sommer, G. Mostoslavsky, D. L. Kaplan, Y.-N. Wu, C.-P. Zhang, L. Wang and J. Chen, Biomaterials, 2011, 32, 5065–5076 CrossRef CAS PubMed.
- D. Zou, Z. Zhang, J. He, S. Zhu, S. Wang, W. Zhang, J. Zhou, Y. Xu, Y. Huang, Y. Wang, W. Han, Y. Zhou, S. Wang, S. You, X. Jiang and Y. Huang, Biomaterials, 2011, 32, 9707–9718 CrossRef CAS PubMed.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, R. S. Tuan and C. Zhang, Biomaterials, 2012, 33, 7008–7018 CrossRef CAS PubMed.
- Y. Deng, H. Zhou, D. Zou, Q. Xie, X. Bi, P. Gu and X. Fan, Biomaterials, 2013, 34, 6717–6728 CrossRef CAS PubMed.
- L. Tortet, J. R. Gavarri, G. Nihoul and A. J. Dianoux, J. Solid State Chem., 1997, 132, 6–16 CrossRef CAS.
- D. Tadic and M. Epple, Biomaterials, 2004, 25, 987–994 CrossRef CAS.
- C. B. Baddiel and E. E. Berry, Spectrochim. Acta, 1966, 22, 1407–1416 CrossRef CAS.
- D. Walsh, T. Furuzono and J. Tanaka, Biomaterials, 2001, 22, 1205–1212 CrossRef CAS.
- K. Rezwan, Q. Z. Chen, J. J. Blaker and A. R. Boccaccini, Biomaterials, 2006, 27, 3413–3431 CrossRef CAS PubMed.
- J. M. Holzwarth and P. X. Ma, Biomaterials, 2011, 32, 9622–9629 CrossRef CAS PubMed.
- M. N. Rahaman, D. E. Day, B. S. Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Acta Biomater., 2011, 7, 2355–2373 CrossRef CAS PubMed.
- Y. S. Nam and T. G. Park, Biomaterials, 1999, 20, 1783–1790 CrossRef CAS.
- A. Salerno, S. Zeppetelli, E. D. Maio, S. Iannace and P. A. Netti, Compos. Sci. Technol., 2010, 70, 1838–1846 CrossRef CAS PubMed.
- J. A. Inzana, D. Olvera, S. M. Fuller, J. P. Kelly, O. A. Graeve, E. M. Schwarz, S. L. Kates and H. A. Awad, Biomaterials, 2014, 35, 4026–4034 CrossRef CAS PubMed.
- K. Seunarine, N. Gadegaard, M. Tormen, D. O. Meredith, M. O. Riehle and C. D. W. Wilkinson, Nanomedicine, 2006, 1, 281–296 CrossRef CAS PubMed.
- J. Yang, T. Long, N.-F. He, Y.-P. Guo, Z.-A. Zhu and Q.-F. Ke, J. Mater. Chem. B, 2014, 2, 6611–6618 RSC.
- N. He, Q. Ke, C. Huang, J. Yang and Y. Guo, J. Appl. Polym. Sci., 2014, 131, 40404 Search PubMed.
- M. S. Abu Bakar, M. H. W. Cheng, S. M. Tang, S. C. Yu, K. Liao, C. T. Tan, K. A. Khor and P. Cheang, Biomaterials, 2003, 24, 2245–2250 CrossRef CAS.
- Z. Shi, X. Huang, Y. Cai, R. Tang and D. Yang, Acta Biomater., 2009, 5, 338–345 CrossRef CAS PubMed.
- S. S. A. Abidi and Q. Murtaza, J. Mater. Sci. Technol., 2014, 30, 307–310 CAS.
- X. Deng, J. Hao and C. Wang, Biomaterials, 2001, 22, 2867–2873 CrossRef CAS.
- W. L. Suchanek, P. Shuk, K. Byrappa, R. E. Riman, K. S. TenHuisen and V. F. Janas, Biomaterials, 2002, 23, 699–710 CrossRef CAS.
- S. Diederichs, K. M. Shine and R. S. Tuan, BioEssays, 2013, 35, 220–230 CrossRef CAS PubMed.
- D. Marolt, M. Knezevic and G. V. Novakovic, Stem Cell Res. Ther., 2010, 1, 10 CrossRef PubMed.
Footnote |
| † J.Guan and J. Yang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |