Fabrication, characterization and photocatalytic activity of g-C3N4 coupled with FeVO4 nanorods
Qingyan Nonga,
Min Cuia,
Hongjun Linc,
Leihong Zhaob and
Yiming He*a
aDepartment of Materials Physics, Zhejiang Normal University, Jinhua, 321004, China. E-mail: hym@zjnu.cn; Fax: +86-0579-82282243; Tel: +86-0579-82282243
bInstitute of Physical Chemistry, Zhejiang Normal University, Jinhua, 321004, China
cCollege of Geography and Environmental Sciences, Zhejiang Normal University, Jinhua, 321004, China
First published on 16th March 2015
Abstract
A novel FeVO4/g-C3N4 composite photocatalyst was synthesized via a simple mixing-calcination method and characterized by various techniques including the Brunauer–Emmett–Teller method, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, UV-vis diffuse reflectance spectroscopy, photoluminescence spectroscopy, and an electrochemical method. The photocatalytic activity was evaluated by degradation of rhodamine B (RhB). Results indicated that the FeVO4/g-C3N4 composite exhibited a much higher photocatalytic activity than pure g-C3N4 under visible light illumination. The rate constant of RhB degradation for the optimal FeVO4/g-C3N4 composite (5.0% FeVO4/g-C3N4) is approximately 2.2 times that of pure g-C3N4. The formation of a heterojunction structure between FeVO4 and g-C3N4 which efficiently promoted the separation of electron–hole pairs was believed to be the origin of the enhanced photoactivity.
1. Introduction
Photocatalytic degradation of organic pollutants by a semiconductor as photocatalyst has attracted considerable interest in the past decades.1,2 The development of an efficient photocatalyst under visible light is considered as the key factor for its practical application, which has triggered scientists to make great effort to design novel visible-light-driven photocatalysts. A great number of semiconductor photocatalysts, such as Ag3PO4,3 BiVO4,4 g-C3N4,5 and CaBi2O4,6 were thus been reported. Of these well-known semiconductors, g-C3N4 has been proven to be the most representative and extensively used material owing to the following merits.7,8 First, it has a band gap of approximately 2.70 eV, which fulfills the basic requirements as a photocatalyst for water splitting, CO2 photoreduction, and organic pollutant decomposition under visible light irradiation. Second, g-C3N4 has high thermal and chemical stability. Finally, g-C3N4 is a polymeric metal-free semiconductor and can be prepared easily, indicating it is low cost and abundant. Nevertheless, the low surface area, the fast recombination of photoexcited electron–hole pairs, and the lack of absorption above 460 nm still limit the photocatalytic activity of g-C3N4. More and more researcher recognized that pure g-C3N4 is hardly competent for efficient solar energy conversion.Meanwhile, heterojunctions that are formed between different semiconductor materials could provide great potential driving force to improve the separation of electron–hole pairs. This finding inspires many scientists to enhance the photocatalytic activity of g-C3N4 via the approach of heterojunction construction. Up to date, a great variety of g-C3N4 based composite photocatalysts have been reported, such as LnVO4 (Ln = Sm, Dy, La)/g-C3N4,9–11 TaON/g-C3N4,12 CdS/g-C3N4,13 AgX (X = Cl, Br, I)/g-C3N4,14,15 ZnO/g-C3N4 (ref. 16) and BiOX (X = Cl, Br, I)/g-C3N4.17–19 Among them, metal vanadate semiconductors show great promotion effect due to their suitable band potential and visible light responsibility. For example, Li et al. reported that the doping of SmVO4 on g-C3N4 increased the photocatalytic activity by a factor of two.9 Wang et al. used Ag3VO4 to modify g-C3N4, yielding an enhanced photoactivity on the composite that was eleven that of pure g-C3N4 and nearly six times higher than pure Ag3VO4.20 Qu et al. synthesized BiVO4/g-C3N4 composite by a simple mixing and calcination method. The prepared composite was proved to be extraordinary more active than that of pure g-C3N4.21 These results indicate that combing metal vanadate with g-C3N4 may provide a feasible way to synthesize an efficient photocatalyst.
In this work, FeVO4 was chosen as a modifier to promote the photocatalytic activity of g-C3N4. FeVO4 is a narrow band gap semiconductor and shows inherent visible light absorption onset at about 600 nm, which facilitates to collect around 45% of the incident solar spectrum energy. Meanwhile, FeVO4 has been reported to show good photocatalytic activity under visible light irradiation.22–24 Zhao et al. synthesized ultrathin FeVO4 nanosheets and applied it in photodegradation of methyl orange.22 Ozturk et al. synthesized surfactant-assisted FeVO4 nanostructure and found that the prepared sample exhibited good activity in phenol degradation.23 Min et al. reported that the coupling of FeVO4 with TiO2 could result in an enhanced photoactivity in degradation of RhB.24 However, to the best of our knowledge, no research focused on the photocatalytic activity of FeVO4/g-C3N4 composite has been reported. This paper found that the coupling of FeVO4 markedly increased the activity of g-C3N4 in RhB degradation. With the help of a thorough investigation of the composite's structure, surface area and optical properties, the origin of the higher photoactivity of the FeVO4/g-C3N4 composite was documented.
2. Experimental section
2.1 Catalysts preparation
All these reagents were analytical pure grade and used without further purification. Pure g-C3N4 powders were prepared by heating melamine at 520 °C for 4 h at a heating rate of 3 °C min−1 in a semi-closed alumina crucible with a cover. Pure FeVO4 nanorod was prepared by a hydrothermal method.22 In a typical synthesis, 2 mmol FeCl3 and 2 mmol NH4VO3 were dissolved in 80 mL deionized water under magnetic stirring at room temperature. After stirring for 30 min, the pH value was adjusted to 3 using HCl solution. Then, the resulting mixture was transferred into a Teflon-lined stainless-steel autoclave and heated to 180 °C for 24 h. After the autoclave was cooled to room temperature, the resulting products were collected, washed with distilled water several times, and dried at 60 °C in air.The FeVO4/g-C3N4 composites were prepared according to the following procedure. A given amount of FeVO4 and g-C3N4 were mixed and ground in an agate mortar for 20 min. Then, the mixture was calcined at 400 °C for 2 h to obtain the FeVO4/g-C3N4 catalyst. By this means, the FeVO4/g-C3N4 composites with different FeVO4 content were prepared.
2.2 Photocatalytic reaction
The photocatalytic activity of FeVO4/g-C3N4 composite for RhB decolorization was evaluated under visible-light irradiation using a 350 W Xe lamp with two cut-off filter (800 nm > λ > 420 nm) as the light source. Experiments were conducted at ambient temperature and procedures were as follows: 100 mL of RhB solution (20 mg L−1) and 0.1 g of photocatalyst were added to a 250 mL Pyrex glass cell. Then, the suspension was agitated for an hour to ensure the adsorption–desorption equilibrium between the catalysts and the dye. After that, the light is turned on and motivates the photodecolorization process of RhB solution. At regular intervals, samples were withdrawn and centrifuged to remove photocatalyst for analysis. The concentration of aqueous RhB was determined by measuring its absorbance of the solution at 554 nm using a UV-vis spectrophotometer. The examination experiment of active species is similar to the photodegradation experiment. A quantity of scavengers was introduced into the RhB solution prior to addition of the catalyst.25,26 Chemical oxygen demand (COD) was measured according to the standard dichromate titration method, using a dichromate solution as the oxidizing agent in a strong acid medium.2.3 Characterizations
The Brunauer–Emmett–Teller (BET) specific surface areas of the catalysts were measured by nitrogen adsorption on Autosorb-1 (Quantachrome Instruments). The powder X-ray diffraction (XRD, Philips PW3040/60) was used to record the diffraction patterns of photocatalysts employing Cu Kα radiation (40 kV/40 mA). The thermogravimetric (TG, Netzsch STA449) analysis of samples were performed from 50 to 800 °C in a flow of air (10 mL min−1) at a heating rate of 10 °C min−1. Scanning electron microscopy (SEM) pictures were taken on a field emission scanning electron microscope (LEO-1530). Transmission electron microscope (TEM) observations were carried out on a JEM-2010F transmission electron microscope at an accelerating voltage of 200 kV. X-Ray photoelectron spectroscopy (XPS) was performed on a Quantum 2000 Scanning ESCA Microprobe instrument using Al Kα. The C 1s signal was set to a position of 284.6 eV. Diffuse reflectance spectroscopy (DRS) was performed on a UV-vis spectrometer (PerkinElmer Lambda900) equipped with an integrating sphere. Photoluminescence spectra (PL) were collected on FLS-920 spectrometer (Edinburgh Instrument), using a Xe lamp (excitation at 365 nm) as light source. The electrochemical impedance spectroscopy (EIS) responses measurements were performed by using a CHI 660B electrochemical workstation with a standard three-electrode cell at room temperature.3. Results and discussion
3.1 Characterizations of FeVO4/g-C3N4 composites
Although pure g-C3N4 exhibits well stability in air below 550 °C, the oxidation process of g-C3N4 is usually accelerated when the coupled semiconductor has the capability of activating oxygen.9 Therefore, it is necessary to detect the real g-C3N4 content for g-C3N4 based composite. Fig. 1 shows the TG profiles of g-C3N4 and FeVO4/g-C3N4 composite with different FeVO4 content. It can be observed that FeVO4/g-C3N4 composite presents a sharp weight loss occurring from 480 to 600 °C, which is much lower than that of pure g-C3N4. This result accords well with the previous report.9,10 The real concentration of FeVO4 can be calculated from the residuals after the samples were heated over 600 °C. It can be concluded that the synthesized FeVO4/g-C3N4 composites has the FeVO4 concentrations of 3.0 wt%, 5.0 wt%, 7.8 wt%, and 9.8 wt%.XRD patterns of FeVO4/g-C3N4 composite photocatalysts with different FeVO4 concentration are shown in Fig. 2. The diffraction peaks at 2θ = 27.4° and 13.4° can be ascribed to (002) and (100) planes of g-C3N4 (JCPDS 87-1526), which correspond to the interplanar staking peaks of aromatic systems and the interlayer structural packing, respectively.27 For FeVO4, the diffraction peaks are identical with the reported data of a triclinic phase in the JCPDS (38-1372). The XRD patterns of FeVO4/g-C3N4 reveal coexistence peaks of FeVO4 and g-C3N4 in the composite. With the content of FeVO4 increasing from 3.8 to 9.7 wt%, the diffraction peaks of FeVO4 increase and the diffraction peaks of g-C3N4 decrease gradually. No other new crystal phases in the composite are found.
The optical properties of g-C3N4, FeVO4 and FeVO4/g-C3N4 were investigated by DRS, and the result is shown in Fig. 3. It can be observed that absorbance threshold of g-C3N4 is located at approximately 460 nm, whereas the FeVO4 sample can absorb light with a wavelength lower than 620 nm. The band gaps of g-C3N4 and FeVO4 can be estimated to be 2.7 eV and 1.98 eV, respectively, according to the Kubelka–Munk equation. Due to the narrow band gap, the introduction of FeVO4 into g-C3N4 clearly improves the photoabsorption performance of the composite. With the FeVO4 content increasing, the FeVO4/g-C3N4 displays an increased property in visible light absorption, which is beneficial for the photocatalytic reaction.
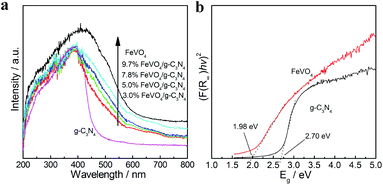 | ||
| Fig. 3 UV-vis spectra of g-C3N4, FeVO4 and FeVO4/g-C3N4 composite with different FeVO4 concentrations (a), and the estimated band gap of FeVO4 and g-C3N4 (b). | ||
Fig. 4 shows the SEM images of pure g-C3N4, FeVO4, and 5.0% FeVO4/g-C3N4 composite. It is revealed that the appearance of FeVO4 is regular nanorod structure with a uniform diameter of ∼100 nm and length of up to 1–2 micrometers. Pure g-C3N4 displays aggregated morphologies, which are comprised of block-based flakiness and particles. For the FeVO4/g-C3N4 composite, the morphology shows the combination of FeVO4 and g-C3N4. Due to their special morphologies of the two semiconductors, it is easily observed that the FeVO4 nanorods have randomly deposited and distributed on the surface of g-C3N4, and formed the composite structures, which accords well the XRD result.
High-resolution transmission electron microscopy (HRTEM) analysis was performed to get information on the microstructure of FeVO4/g-C3N4. Fig. 5 shows the low- and high-magnification TEM images of the 5.0% FeVO4/g-C3N4. Given the different morphology and molecular weight of FeVO4 and g-C3N4, the dark parts in the TEM image (Fig. 5a) should be FeVO4, while the light parts correspond to g-C3N4. It can be observed that FeVO4 nanorods disperse in g-C3N4, which agrees well with the SEM result. Meanwhile, considering that the FeVO4/g-C3N4 hybrids were ultrasonicated for 20 min before TEM analysis, the result in Fig. 5 indicates the strong interaction between FeVO4 and g-C3N4 in the hybrids. The clear lattice fringe of 0.3609 nm in the HRTEM image of FeVO4/g-C3N4 should be ascribed to the (100) planes of FeVO4. Smooth and intimate interfaces are clearly observed between the g-C3N4 and FeVO4, which confirms the formation of FeVO4/g-C3N4 heterojunction.28,29 The observed interface is possible favorable for the transport of photogenerated carriers, and thereby promotes the separation of electron–hole pairs.
The surface chemical compositions and states of the g-C3N4, FeVO4, and FeVO4/g-C3N4 were investigated by XPS. The survey XPS spectra (Fig. 6a) indicates that C 1s and N 1s peaks are detected for g-C3N4 and FeVO4/g-C3N4, while Fe 2p, V 3d, and O 1s peaks are displayed for FeVO4 and FeVO4/g-C3N4. This result is consistent with the chemical composition of the photocatalysts. Fig. 6b shows the high-resolution X-ray photoelectron spectra (HRXPS) of C 1s. Pure g-C3N4 shows two peaks at 284.6 eV and 287.8 eV, which can be assigned to the adventitious carbon and the sp2-bonded carbon in N-containing aromatic rings (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), respectively.30 The FeVO4/g-C3N4 composite also display the two C 1s peaks. However, compared with pure g-C3N4, the peak corresponding to the g-C3N4 shows a slightly positive shift. This kind shift is also observed in the N 1s XPS spectra (Fig. 6c), and can be ascribed to the fact that FeVO4 hybridized with g-C3N4. Indeed, the hybridization of FeVO4 and g-C3N4 influence not only the C 1s and N 1s spectra, but also the Fe 2p and V 2p spectra. As shown in Fig. 6d, the V 2p3/2 and 2p1/2 peaks of the FeVO4 are located at 516.9 eV and 525.2 eV, respectively, corresponding to V5+ in FeVO4.31 The BE of Fe 2p3/2 and 2p1/2 of the FeVO4 is determined to be 711.2 eV and 725.3 eV, respectively, indicating the 3+ valence of Fe.32 Both of them are slightly lower than those of FeVO4/g-C3N4. The XPS results prove the strong interaction between FeVO4 and g-C3N4, which accords well with the TEM result. Fig. 6f shows the VB XPS spectra of g-C3N4 and FeVO4. The positions of the VB edges of g-C3N4 and FeVO4 are determined to be 1.50 and 1.76 eV, respectively.
N), respectively.30 The FeVO4/g-C3N4 composite also display the two C 1s peaks. However, compared with pure g-C3N4, the peak corresponding to the g-C3N4 shows a slightly positive shift. This kind shift is also observed in the N 1s XPS spectra (Fig. 6c), and can be ascribed to the fact that FeVO4 hybridized with g-C3N4. Indeed, the hybridization of FeVO4 and g-C3N4 influence not only the C 1s and N 1s spectra, but also the Fe 2p and V 2p spectra. As shown in Fig. 6d, the V 2p3/2 and 2p1/2 peaks of the FeVO4 are located at 516.9 eV and 525.2 eV, respectively, corresponding to V5+ in FeVO4.31 The BE of Fe 2p3/2 and 2p1/2 of the FeVO4 is determined to be 711.2 eV and 725.3 eV, respectively, indicating the 3+ valence of Fe.32 Both of them are slightly lower than those of FeVO4/g-C3N4. The XPS results prove the strong interaction between FeVO4 and g-C3N4, which accords well with the TEM result. Fig. 6f shows the VB XPS spectra of g-C3N4 and FeVO4. The positions of the VB edges of g-C3N4 and FeVO4 are determined to be 1.50 and 1.76 eV, respectively.
 | ||
| Fig. 6 XPS spectra of g-C3N4, FeVO4 and 5.0% FeVO4/g-C3N4 composite (a) survey spectra (b) C 1s, (c) N 1s (d) V 2p, (e) Fe 3p, (f) VB XPS spectra of FeVO4 and g-C3N4. | ||
The specific surface area of FeVO4/g-C3N4 composite was investigated by N2 adsorption method. Pure FeVO4 exhibits a BET surface area of 13.3 m2 g−1, which is slightly higher than that of pure g-C3N4 (11.2 m2 g−1). For the samples of 3.0% FeVO4/g-C3N4, 5.0% FeVO4/g-C3N4, 7.8% FeVO4/g-C3N4 and 9.8% FeVO4/g-C3N4, the BET values are equal to 12.7, 17.1, 15.3, 19.0 m2 g−1, respectively. These values are in the same order of magnitude, indicating that the addition of FeVO4 to g-C3N4 affects the BET area slightly. Meanwhile, no correlation between the FeVO4 content and the BET value is observed.
3.2 Photocatalytic activities of FeVO4/g-C3N4
Due to RhB is a common contaminant in industry wastewater, the photocatalytic oxidation of RhB was used to investigate the photocatalytic activity of FeVO4/g-C3N4 composites. Fig. 7 shows the photocatalytic activity of g-C3N4, FeVO4 and FeVO4/g-C3N4 with different FeVO4 concentration. The blank test indicates that the photolysis of RhB is negligible. Pure FeVO4 shows a low photocatalytic activity. The reaction rate constant (k) is estimated to be 0.002 min−1, which is only 1/5 of that of pure g-C3N4. The addition of FeVO4 on g-C3N4 enhances the photocatalytic activity. The photocatalytic activity of FeVO4/g-C3N4 increases with the increase in the FeVO4 content up to 5.0%. When the FeVO4 content is higher than 5.0%, the photoactivity of the sample decreases gradually. It is clear that the amount of FeVO4 has an important influence on the photocatalytic activity of the samples. The reason may be that when the amount of FeVO4 is higher than 5.0%, the excess FeVO4 on the surface of g-C3N4 may hinder light absorption of g-C3N4 and cover the active sites in g-C3N4, thereby the photocatalytic activity of the samples is decreased. Under the experimental conditions, the optimal concentration of FeVO4 in FeVO4/g-C3N4 is 5.0 wt%. The 5.0% FeVO4/g-C3N4 presents the highest photocatalytic activity with a k of 0.022 min−1, which is 2.2 times higher than that of pure g-C3N4. For comparative purpose, the photocatalytic activity of FeVO4/g-C3N4 mixture was also investigated, and the result is shown in Fig. 7. It can be observed that the photoactivity of 5.0% FeVO4/g-C3N4–PM is much lower than that of 5.0% FeVO4/g-C3N4, indicating the synthesized hybrid is different from a physical mixture, as proved by the XPS and TEM experiments. | ||
| Fig. 7 Photocatalytic activity of FeVO4/g-C3N4 composite with different FeVO4 concentration (a) and the corresponding first-order kinetics plot (b). | ||
Although the data in Fig. 7 has proven the high photocatalytic activity of FeVO4/g-C3N4 composite, it should be noted that the obtained photoactivity means the catalyst's ability in dye decolorization. In other words, it is unknown whether the decolorization RhB was completely degraded to inorganic compounds. In order to resolve this issue, COD measurements of the RhB solution were also performed. The result shows a COD removal of 68.4% over 5% FeVO4/g-C3N4 sample, which is slightly lower than that of color removal. Considering that the color is removed faster that of COD over many photocatalysts,33–35 the synthesized FeVO4/g-C3N4 hybrid can be seen as an efficient photocatalyst.
In addition to the photocatalytic activity, the catalyst's lifetime is another important parameter. Therefore, it is essential to evaluate the stability of the catalyst for practical application. 5.0% FeVO4/g-C3N4 photocatalyst was chosen to as a sample to evaluate the stability of FeVO4/g-C3N4 composite. Fig. 8 shows the photocatalytic activity of 5.0% FeVO4/g-C3N4 sample during the repetition tests. Result suggests that there is no obvious decrease in the photocatalytic efficiency of RhB after five times experiments, which indicates that FeVO4/g-C3N4 has a good stability in the photocatalytic reaction process.
3.3 Possible mechanism of FeVO4/g-C3N4 composite
One can see that, although the photoabsorption performance of the FeVO4/g-C3N4 composite increases with the FeVO4 content increasing, the photocatalytic activity of the samples does not. The similar phenomenon occurs on the adsorption of RhB. In general, a high adsorption of RhB is desired for the photocatalytic reaction. In the case of FeVO4/g-C3N4 composite, all samples present similar ability in RhB adsorption (not shown here), which may be attributed to their similar BET surface areas. The result implies that both the optical property and the adsorption capability of the samples are not the key factor that influences the photocatalytic activity. There should be another crucial factor. Indeed, besides the optical absorption and surface area, the efficient charge separation of a semiconductor usually plays a crucial role in determining its photocatalytic property.36 Especially for the composite photocatalysts, the enhanced separation efficiency via the charge transfer between different semiconductors has been believed to be the most important reason for their high photocatalytic activity. Therefore, the band potentials of g-C3N4 and FeVO4 were investigated in order to clarify the charge separation in FeVO4/g-C3N4 composite. The VB edges of FeVO4 and g-C3N4 have been determined to be 1.76 eV and 1.50 eV, respectively, via the VB XPS experiment. By the equation of ECB = EVB − Eg, the CB edge potentials of FeVO4 and g-C3N4 can be calculated to be −0.22 eV and −1.2 eV, respectively. On the basis of the above results and previous reports,37–39 a possible mechanism for the enhanced photocatalytic activity over the present FeVO4/g-C3N4 is shown in Fig. 9. Under visible light irradiation, both FeVO4 and g-C3N4 can be excited and generate electron–hole pairs. Since the CB edge potential of g-C3N4 is more negative than that of FeVO4, the photoexcited electrons on g-C3N4 can transfer to the CB of FeVO4. Meanwhile, the photogenerated holes on FeVO4 can migrate to the VB of g-C3N4. In such a way, the photoexcited electron–hole pairs could be effectively separated, which suppresses the recombination of electron–hole pairs and subsequently results in an enhanced photocatalytic activity of the FeVO4/g-C3N4 heterojunctions.In order to support aforementioned mechanism, PL experiment was performed to analyze the electron–hole pairs' recombination in FeVO4/g-C3N4 composite. Based on the reported literatures,40,41 it could be known that the higher PL intensity usually means more recombination of electron–hole pairs and lower photocatalytic activity. As shown in Fig. 10, pure g-C3N4 exhibits a strong emission at about 460 nm, which is similar to the previous reports.13,14 Once FeVO4 was added, the emission intensity of PL spectra for the FeVO4/g-C3N4 composite is greatly decreased. Actually, the weakened PL emission is also observed on the physical mixture of FeVO4 and g-C3N4 (5.0% FeVO4/g-C3N4–PM). However, the PL peak of the 5.0% FeVO4/g-C3N4–PM is still much higher than that of 5.0% FeVO4/g-C3N4 composite. For the FeVO4/g-C3N4 physical mixture, the weaken PL emission can be attributed to that less light reaches g-C3N4 due to the decreased g-C3N4 content. For the FeVO4/g-C3N4 composite, the weaker PL peak when compared to the 5.0% FeVO4/g-C3N4–PM definitely indicates that the composite has a much lower recombination rate of electron–hole pairs, which is consistent with the aforementioned mechanism.
In addition to PL analysis, the EIS experiment was also carried out to investigate the separation efficiency of electron–hole pairs of the FeVO4/g-C3N4 composite. Fig. 11 shows the EIS changes of g-C3N4, FeVO4 and 5.0% FeVO4/g-C3N4 composite. The arc size of the three electrodes is 5.0% FeVO4/g-C3N4 < FeVO4 < g-C3N4. In general, the decreased semicircle diameter indicates the smaller charge-transfer resistance on the electrode surface which results in an effective electron–hole separation.42,43 The result in Fig. 11 indicates that the FeVO4/g-C3N4 composite exhibits lower interfacial charge-transfer resistance, which would greatly improve carrier transport efficiency and is beneficial for reducing the recombination rate of photogenerated electrons and holes. This result agrees well the PL analysis and the suggested mechanism in Fig. 9.
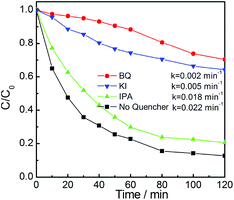
The reactive species trapping experiment was performed to further investigate the active species in the photocatalytic reaction on FeVO4/g-C3N4 hybrid. Fig. 12 shows the photocatalytic activity of 5% FeVO4/g-C3N4 with different quenchers. It can be observed that the addition of benzoquinone (BQ, quencher of ˙O2−) leads to a significantly inactivity of the composite. The reaction constant decreases from 0.022 to 0.002 min−1, indicating ˙O2− is the main reactive species in the photocatalytic process.25,26 The addition of kalium iodide (KI, quencher of h+ and ˙OH) can also dramatically decrease the photocatalytic activity, whereas isopropanol (IPA, quencher of ˙OH) just shows a slight effect on the reaction of RhB photodegradation.25,26 This result implies that h+ plays an more important role than ˙OH species. The data in Fig. 12 indicates that ˙O2− and h+ are two main reactive species in the photocatalytic process of FeVO4/g-C3N4 photocatalyst.
4. Conclusion
A novel FeVO4/g-C3N4 composite photocatalyst was prepared by a simple milling and calcination method. The synthesized FeVO4/g-C3N4 composite show obviously enhanced photocatalytic activity in RhB degradation under visible light irradiation. Such a remarkable enhancement of photocatalytic efficiency is mainly ascribed to the match of conduction and valence band levels between the g-C3N4 and the FeVO4, which can induce the high separation of photogenerated electron–hole pairs in the heterojunction system. This work demonstrates that the combination of FeVO4 and g-C3N4 may be an ideal system for practical application in environmental purification.Acknowledgements
We acknowledge Dr Xiaodong Yi in Xiamen University for his help in XPS analysis. This work was financially supported financially supported by Natural Science Foundation of Zhejiang Province in China (LY14B030002).Notes and references
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- S. Y. Dong, J. L. Feng, M. H. Fan, Y. Q. Pi, L. M. Hu, M. L. Liu, J. Y. Sun and J. H. Sun, RSC Adv., 2015, 5, 14610–14630 RSC.
- Y. P. Bi, S. X. Ouyang, N. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- S. M. Sun and W. Z. Wang, RSC Adv., 2014, 4, 47136–47152 RSC.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- J. W. Tang, Z. G. Zou and J. H. Ye, Angew. Chem., Int. Ed., 2004, 43, 4463–4466 CrossRef CAS PubMed.
- Z. W. Zhao, Y. J. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- J. J. Zhu, P. Xiao, H. L. Li and S. A. C. Carabineiro, ACS Appl. Mater. Interfaces, 2014, 6, 16449–16465 CAS.
- T. T. Li, L. H. Zhao, Y. M. He, J. Cai, M. F. Luo and J. J. Lin, Appl. Catal., B, 2013, 129, 255–263 CrossRef CAS PubMed.
- Y. M. He, J. Cai, T. T. Li, Y. Wu, Y. M. Yi, L. H. Zhao and M. F. Luo, Ind. Eng. Chem. Res., 2012, 51, 14729–14737 CrossRef CAS.
- Y. M. He, J. Cai, L. H. Zhang, X. X. Wang, H. J. Lin, B. T. Teng, L. H. Zhao, W. Z. Weng, H. L. Wan and M. H. Fan, Ind. Eng. Chem. Res., 2014, 53, 5905–5915 CrossRef CAS.
- S. C. Yan, S. B. Lv, Z. S. Li and Z. G. Zou, Dalton Trans., 2010, 39, 1488–1491 RSC.
- J. Y. Zhang, Y. H. Wang, J. Jin, J. Zhang, Z. Lin, F. Huang and J. G. Yu, ACS Appl. Mater. Interfaces, 2013, 5, 10317–10324 CAS.
- H. Xu, J. Yan, Y. G. Xu, Y. H. Song, H. M. Li, J. X. Xia, C. J. Huang and H. L. Wan, Appl. Catal., B, 2013, 129, 182–193 CrossRef CAS PubMed.
- J. Yan, H. Xu, Y. G. Xu, C. Wang, Y. H. Song, J. X. Xia and H. M. Li, J. Nanosci. Nanotechnol., 2014, 14, 6809–6815 CrossRef CAS PubMed.
- Y. J. Wang, R. Shi, J. Lin and Y. F. Zhu, Energy Environ. Sci., 2011, 4, 2922–2929 CAS.
- Y. Bai, P. Q. Wang, J. Y. Liu and X. J. Liu, RSC Adv., 2014, 4, 19456–19461 RSC.
- J. Di, J. X. Xia, S. Yin, H. Xu, L. Xu, Y. G. Xu, M. Q. He and H. M. Li, J. Mater. Chem. A, 2014, 2, 5340–5351 CAS.
- J. Di, J. X. Xia, S. Yin, H. Xu, M. Q. He, H. M. Li, L. Xu and Y. P. Jiang, RSC Adv., 2013, 3, 19624–19631 RSC.
- S. M. Wang, D. L. Li, C. Sun, S. G. Yang, Y. Guan and H. He, Appl. Catal., B, 2014, 144, 885–892 CrossRef CAS PubMed.
- M. Qu, Q. Zhong and S. L. Zhang, J. Sol-Gel Sci. Technol., 2014, 72, 443–454 CrossRef.
- Y. Zhao, K. Yao, Q. Cai, Z. J. Shi, M. Q. Sheng, H. Y. Lin and M. W. Shao, CrystEngComm, 2014, 16, 270–276 RSC.
- B. Ozturk and G. S. P. n. Soylu, J. Mol. Catal. A: Chem., 2015, 398, 65–71 CrossRef CAS PubMed.
- Y. L. Min, K. Zhang, Y. C. Chen and Y. G. Zhang, Chem. Eng. J., 2011, 175, 76–83 CrossRef CAS PubMed.
- Y. M. He, J. Cai, T. T. Li, Y. Wu, H. J. Lin, L. H. Zhao and M. F. Luo, Chem. Eng. J., 2013, 215–216, 721–730 CrossRef CAS PubMed.
- G. T. Li, K. H. Wong, X. W. Zhang, C. Hu, J. C. Yu, R. C. Y. Chan and P. K. Wong, Chemosphere, 2009, 76, 1185–1191 CrossRef CAS PubMed.
- J. F. Zhang, Y. F. Hu, X. L. Jiang, S. F. Chen, S. G. Meng and X. L. Fu, J. Hazard. Mater., 2014, 280, 713–722 CrossRef CAS PubMed.
- H. F. Shi, G. Q. Chen, C. L. Zhang and Z. G. Zou, ACS Catal., 2014, 4, 3637–3643 CrossRef CAS.
- X. Zong, H. J. Yan, G. P. Wu, G. J. Ma, F. Y. Wen, L. Wang and C. Li, J. Am. Chem. Soc., 2008, 130, 7176–7177 CrossRef CAS PubMed.
- H. J. Yan, Y. Chen and S. M. Xu, Int. J. Hydrogen Energy, 2012, 37, 125–133 CrossRef CAS PubMed.
- W. Yang, G. Q. Tan, J. Huang, H. J. Ren, A. Xia and C. C. Zhao, Ceram. Int., 2015, 41, 1495–1503 CrossRef CAS PubMed.
- S. K. Biswas and J. O. Baeg, Int. J. Hydrogen Energy, 2013, 38, 14451–14457 CrossRef CAS PubMed.
- X. C. Zhang, X. X. Liu, C. M. Fan, Y. W. Wang, Y. F. Wang and Z. H. Liang, Appl. Catal., B, 2013, 132–133, 332–341 CrossRef CAS PubMed.
- T. Essama, M. A. Amin, O. E. Tayeb, B. Mattiasson and B. Guieysse, Water Res., 2007, 41, 1697–1704 CrossRef PubMed.
- W. Z. Yin, W. Z. Wang, L. Zhou, S. M. Sun and L. Zhang, J. Hazard. Mater., 2010, 173, 194–199 CrossRef CAS PubMed.
- X. J. Guan and L. J. Guo, ACS Catal., 2014, 4, 3020–3026 CrossRef CAS.
- S. Kumar, S. Khanchandani, M. Thirumal and A. K. Ganguli, ACS Appl. Mater. Interfaces, 2014, 6, 13221–13233 CAS.
- A. Kumar, T. Surendar, A. Baruah and V. Shanker, J. Mater. Chem. A, 2013, 1, 5333–5340 Search PubMed.
- L. Q. Jing, Y. C. Qu, B. Q. Wang, S. D. Li, B. J. Jiang, L. B. Yang, W. Fu, H. G. Fu and J. Z. Sun, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef CAS PubMed.
- L. Y. Huang, Y. P. Li, H. Xu, Y. G. Xu, J. X. Xia, K. Wang, H. M. Li and X. N. Cheng, RSC Adv., 2013, 3, 22269–22279 RSC.
- P. Q. Li, X. N. Sui, J. F. Xu, H. Jing, C. X. Wu, H. Peng, J. Lu and H. Z. Yin, Chem. Eng. J., 2013, 234, 361–371 CrossRef PubMed.
- H. T. Yu, X. Quan, S. Chen, H. M. Zhao and Y. B. Zhang, J. Photochem. Photobiol., A, 2008, 200, 301–306 CrossRef CAS PubMed.
- T. G. Xu, L. W. Zhang, H. Y. Cheng and Y. F. Zhu, Appl. Catal., B, 2011, 101, 382–387 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |