In situ synthesis of gold nanoparticles on LBL coated nanofibers by tannic acid for catalytic application†
Abstract
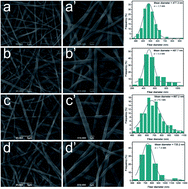
Electrospinning nanofibrous mats are extensively studied as efficient two-dimensional nanomaterials and applied in the fields of filtration, catalysis, and biosensors due to their flexibility and porosity. In this article, gold nanoparticle (AuNPs) loaded composite nanofibers were fabricated by a simple method, which consisted of the preparation of the nanofibers by electrospinning, the deposition of tannic acid (TA) on the surface of the nanofibers via layer-by-layer assembly and the reduction of the AuNPs on the nanofibrous mats. The as-prepared nanofibers were characterized by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS), respectively. The results revealed that AuNPs were successfully generated on the nanofibers without aggregation. In addition, by adjusting the number of the bilayers in the assembly process, the content of gold supported on the nanofibrous mats could be easily controlled. The catalytic performance of the hybrid nanofibrous mats on the reduction of 4-nitrophenol (4-NP) with sodium borohydride was monitored by UV-visible spectroscopy (UV-vis). Notably, the hybrid composite nanofibrous mats could be easily separated from the reaction mixture.

 Please wait while we load your content...
Please wait while we load your content...