Dehydrogenation of propane over PtSn/SBA-15 catalysts: effect of the amount of metal loading and state
Abstract
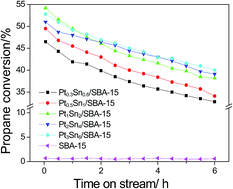
A series of PtSn/SBA-15 catalysts with different metal loading amounts were prepared by an incipient-wetness impregnation method and their catalytic performances were tested for propane dehydrogenation. The catalysts were characterized by means of XRD, BET, TEM, UV-Vis DRS, O2-TPO, H2-TPR, XPS and Raman spectroscopy. The catalytic activity for propane dehydrogenation increases with an increase in the loading amount of metal, and it remained stable when the Pt loading amount reached 1 wt%. It is found that the state of Pt or Sn in the catalysts varies with changes in metal loading. When the loading of Pt and Sn exceed a certain amount (Pt 1 wt% and Sn 2 wt%), the state of Pt and Sn changes apparently with the more oxidative Pt and more reduced Sn. The changes of the state of Pt and Sn influence the catalytic performance of these catalysts. Moreover, the size of the PtSn nanoparticles increases with increasing amounts of Pt and Sn, which also results in a change in the catalytic performance. The Pt1Sn2/SBA-15 catalyst shows the highest initial activity, which results from an increased amount of active sites, the high dispersion of PtSn nanoparticles and the favorable state of the Pt and Sn species.

 Please wait while we load your content...
Please wait while we load your content...