A one-pot method to prepare a ZnO/Ag/polypyrrole composite for zinc alkaline secondary batteries†
Jianhang Huangab and
Zhanhong Yang*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha 410083, PR China. E-mail: zhongnan320@gmail.com; jhhuang@csu.edu.cn
bInnovation, base of energy and chemical materials for graduate students training, Central South University, Changsha 410083, PR China
First published on 26th March 2015
Abstract
A ZnO/Ag/polypyrrole (ZAP) composite was synthesized by a facile one-pot method in which silver ammonia complex ions were reduced to metallic silver using a pyrrole monomer as the reducing agent. Meanwhile, the pyrrole monomer was oxidized and formed polypyrrole. When evaluated as an anode material for zinc alkaline secondary batteries, the composite demonstrated a superior electrochemical performance. This material presented a much more stable cycle performance and better reversibility in a galvanostatic discharge–charge test. These virtues are due to the good conductivity of silver and the trapping effect of polypyrrole, which retain the zincate ions in the vicinity of the electrode rather than dissolving into the electrolyte.
As the most used anode material for alkaline primary batteries, zinc electrodes possess a unique set of advantages such as low equivalent weight, a high specific energy density, abundance and low toxicity, and is the most electropositive metal that is relatively stable in aqueous and alkaline media without significant corrosion.1–5 Due to these virtues of zinc electrodes and because the electrochemical redox reaction of zinc in alkaline electrolytes is easily reversible, many researchers want to apply zinc electrodes to secondary battery systems. Unfortunately, when evaluated as an anode material for secondary batteries, the non-uniform dissolution and deposition of zinc species during extensive charge–discharge cycling usually result in serious electrode dendritic growth and shape change, which are harmful to battery performance and cycle life.6–9 In order to mitigate these problems, many different approaches have been attempted. Among the various approaches, surface modifications have been considered as an effective method to improve the electrochemical performance of electrodes in various battery systems.4,10–15 Lee et al.16 prepared TiO2-coated ZnO by a sol–gel method, which suppressed the dissolution of Zn by forming a passive surface layer. Yang et al.17 prepared In2O3 doped ZnO with a plate-like morphology, which showed low resistivity and higher capacity retention. Other than metal oxides, organic materials are effective for retaining the discharge product on zinc electrodes due to their fine porous structure and re-complexation of zincate ions.18 Vatsalarani et al.19 found that a fibrous network of polyaniline coating allowed the movement of hydroxide ions but restricted the diffusion of zincate ions.
In this study, an attempt has been made to prepare a ZnO/Ag/polypyrrole composite to combine the advantages of metallic and organic modifications. It is well known that silver is an excellent conductor of electricity, plus the re-complexation of zinc species with polypyrrole mitigates dendritic growth and shape change, so it can be expected that the as-prepared composite would present a better cycle performance than pristine ZnO.
Fig. 1a shows the FT-IR spectra of the ZAP composite which is characterized by five obvious absorption bands appearing at 3430, 1623, 1384, 1082 and 492 cm−1. The strong absorption at 3430 cm−1 is assigned to N–H stretching in polypyrrole or O–H stretching in absorbed water.20 Another strong absorption at 492 cm−1 is assigned to the Zn–O bond due to the high content of ZnO in the composite. The other absorptions at 1623, 1384 and 1082 cm−1 are characteristic absorptions, which can be assigned to the vibration of a conjugated double bond in the pyrrole ring, although there is some degree of shift to a higher wavenumber with comparison to pure polypyrrole.21,22 The shift in wavenumber is due to the interaction between polypyrrole and the Ag nanoparticles and influences the skeletal vibrations. This phenomenon can also be observed in other polypyrrole/metal oxide composites. In detail, the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond stretching of the pyrrole ring leads to the appearance of absorption at 1623 cm−1. The absorptions at 1384 and 1082 cm−1 are associated with C–H in-plane vibrations and bending, respectively. These absorption bands demonstrate the existence of polypyrrole in the composite.
C double bond stretching of the pyrrole ring leads to the appearance of absorption at 1623 cm−1. The absorptions at 1384 and 1082 cm−1 are associated with C–H in-plane vibrations and bending, respectively. These absorption bands demonstrate the existence of polypyrrole in the composite.
Fig. 1b shows the XRD patterns of pristine ZnO and the ZAP composite. It is obvious from curve A that the XRD pattern of pristine ZnO matches well with that of standard ZnO with the hexagonal wurtzite structure (JCPDS card 36-1451). As for the ZAP composite, the diffraction peaks corresponding to ZnO can also be observed clearly, indicating that the hexagonal wurtzite structure of ZnO in the ZAP composite had not been changed during the synthesis process. Besides the diffraction peaks for ZnO, the diffraction peaks assigned to metallic silver at a 2θ of 38.0°, 44.3°, 64.5° and 77.3° can be obviously noticed, which proves that the silver–ammonia complex ion had been reduced to metallic silver.
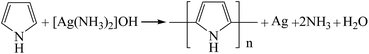
Generally, polypyrrole is usually prepared by an oxidation reaction of pyrrole in a chemical route. The most commonly used oxidizing agents are ferric chloride and ammonium persulfate.23–26 Fe(III) will be reduced to Fe(II) when it oxidizes pyrrole. The standard reduction potential of Fe(III) to Fe(II) is 0.771 V. So in theory, if an oxidizing agent possesses a higher reduction potential than Fe(III), it is capable of oxidizing pyrrole. The standard reduction potential of Ag(I) to Ag(0) is 0.7996 V, so it has the ability to polymerize the pyrrole monomer, and itself would be reduced to metallic silver simultaneously, the presumed reaction equation is presented as follow:
The morphologies of ZnO and the ZAP composite are shown in Fig. 2. It can be seen in Fig. 2a that the pristine ZnO particle presents a hexagonal prism morphology with a smooth surface, and the size of the ZnO particles is about 200–500 nm. Fig. 2b shows the morphology of the ZAP composite, it is obvious that there is some tiny metallic silver particles attached on the surface of the ZnO particles with a uniform distribution. In order to further understand the structure and components of the ZAP composite, TEM was performed and the results can be seen in Fig. 2c. The morphology of the ZAP composite observed in the TEM images is consistent with that in the SEM images, and the size of the metallic silver particles is around the 50 nm. The smaller size of the Ag particles is beneficial to the improvement of electrode conductivity and current distribution, leading to a smaller electrode polarization. What is more important, is that the polypyrrole (PPy) coating film upon the silver surface can be observed in the TEM image (indicated by the arrow in the inset of Fig. 2c), and the interplanar distance of 0.236 nm corresponding to (111) crystal planes for metallic silver can be observed in Fig. 2d, which indicates that the ZnO/Ag/polypyrrole composite has been synthesized successfully. In addition, the specific surface areas of pristine ZnO and the ZAP composite, estimated with the Brunauer–Emmett–Teller (BET) method, are 5.98 and 6.49 m2 g−1, respectively. In principle, a higher surface area of materials is preferred for better electrochemical performance due to the increased active sites.
The galvanostatic discharge–charge analysis at a 1 C rate over a voltage range of 1.2 to 1.9 V was performed in order to investigate the cycle performance curves of the ZnO electrode and the ZAP electrode. As can be seen in Fig. 3a, both the ZnO and ZAP electrodes suffer relative low discharge capacity in the initial few cycles because of an activated process. As for the pristine ZnO (curve A), it reaches the highest discharge capacity rapidly and declines swiftly in subsequent cycles. By comparison, it takes more cycles (about eight cycles) for the ZAP electrode to reach its highest discharge capacity compared to pristine ZnO (about three cycles).
This phenomenon could be ascribed to the fact that silver nanoparticles and polypyrrole modified on the ZnO surface can reduce the contact surface of the ZnO and electrolyte, which prolongs the active process of the ZAP electrode. Although the electrochemical performance of the pristine ZnO electrode in the initial stage of the cycle is better than that of the ZAP electrode, the latter presents a much better capacity retention in succeeding cycles. The capacity retention of the ZAP electrode is measured to be as high as 82% after 100 cycles, while the capacity retention is only 43% for the pristine ZnO electrode. The results obviously indicate that the ZAP composite possesses a better cycle stability than pristine ZnO. It can be explained by the metallic silver and polypyrrole over the surface of the ZnO particles. The excellent conductivity of metallic silver provides small polarization for the ZAP electrode. Furthermore, the polypyrrole plays an important role of a surface trapping layer, which has a re-complexation effect with zincate ions, keeping zincate ions in the vicinity of the electrode rather than dissolving into the electrolyte for pristine ZnO. The advantages of the ZAP electrode is also reflected in the charge–discharge curves. Fig. 3b shows the typical charge–discharge curves at the 10th cycle of pristine ZnO (curve A) and the ZAP electrode (curve B). It is noticeable that the ZAP electrode has a relatively lower charge plateau and a higher discharge plateau compared with the pristine ZnO electrode, which implies that the reversibility of the ZAP electrode is better than that of the pristine ZnO electrode, due to the decrease of the resistance benefited from silver addition.
Electrochemical impedance was employed to investigate the electrode reaction kinetics. The corresponding Nyquist plots of pristine ZnO and the ZAP electrode at the 10th cycle with 100% state-of-charging are shown in Fig. 3c. We can observe that the Nyquist plots are characterized by a semicircle in the higher frequency region (the arc radius represents the magnitude of the charge-transfer resistance) and a straight line in the lower frequency region (Warburg impedance (Zw), which is characteristic of semi-infinite diffusion). A Randles–Ershler type equivalent circuit was adopted to analyze the Nyquist plots. The charge-transfer resistance (Rct) can be calculated from the diameter of the high-frequency arc, which is 6.1 Ω and 1.2 Ω for the pristine ZnO electrode and the ZAP electrode, respectively. The lower Rct demonstrates that the conductivity of the electrode is improved, and the kinetics process of the electrochemical reaction is facilitated. Otherwise, the Warburg impedance for the ZAP electrode is 0.08 Ω, which is larger than 0.04 Ω for pristine ZnO, indicating that the decoration on the surface of the ZnO particles exerts some influence over the diffusion between the solution and the electrode. Taking the two factors into account, the improvement on the initial charge–discharge curves of ZAP is not obvious.
Fig. 3d shows the cyclic voltammetry of pristine ZnO and the ZAP composite at the 10th cycle. It can be seen that the interval between the anode peak and the cathodic peak is 0.325 and 0.275 V for pristine ZnO and the ZAP composite, respectively. The smaller interval indicates that the reversibility of ZAP is better than that of ZnO. But another anode peak was observed around −1.06 V for ZAP, while no similar anode peak was observed for ZnO. This two anode peaks phenomenon is caused by two electrochemical reactions during the discharge process:27
| Zn + 4OH− = Zn(OH)42− + 2e | (1) |
| Zn + 3OH− = Zn(OH)3− + 2e | (2) |
Conclusions
By using a silver ammonia complex ion and a pyrrole monomer as the oxidizing agent and reducing agent respectively, we have been able to directly synthesize a ZnO/Ag/polypyrrole composite by a one-pot method, that presents superior electrochemical performance compared to pristine ZnO. An FT-IR spectrum confirmed the formation of polypyrrole, and the XRD results demonstrated the existence of metallic silver. Furthermore, SEM and TEM images reveal the morphology and structure of the ZAP composite, in which we can see that the small metallic silver particles are coated with polypyrrole, and the polypyrrole coated Ag is decorated upon the surface of the prismatic ZnO particles. When evaluated as an anode material for Zn–Ni secondary batteries, the ZAP electrode exhibits a much higher capacity retention of about 82% than for the pristine ZnO electrode (about 43%) after 100 cycles. In view of the good cycle performance, this material presents a promising application for zinc-based secondary batteries.Acknowledgements
We are grateful for financial support from the Natural Science Foundation of China (no. 21371180), the Doctoral Fund of Ministry of Education of China (20130162110 018) and the Science and Technology Project of Changsha city (no. k1303015-11).Notes and references
- J. Jiri, J. Power Sources, 1997, 66, 15 CrossRef.
- J. Jiri, J. Power Sources, 2000, 88, 202 CrossRef.
- F. R. McLarnon and E. J. Cairns, J. Electrochem. Soc., 1991, 138(2), 645 CrossRef CAS PubMed.
- J. H. Huang and Z. H. Yang, RSC Adv., 2014, 4, 19205 RSC.
- Y. Li and H. Dai, Chem. Soc. Rev., 2014, 43(15), 5257 RSC.
- Z. Feng, Z. Yang, J. Huang, X. Xie and Z. Zhang, J. Electrochem. Soc., 2014, 161, A1981 CrossRef CAS PubMed.
- T. T. Wang, Z. H. Yang, B. Yang, R. J. Wang and J. H. Huang, J. Power Sources, 2014, 257, 174 CrossRef CAS PubMed.
- H. Chang and C. Lim, J. Power Sources, 1997, 66, 115 CrossRef CAS.
- R. K. Ghavami and Z. Rafiei, J. Power Sources, 2006, 162(2), 893 CrossRef CAS PubMed.
- J. H. Huang and Z. H. Yang, ECS Electrochem. Lett., 2014, 3(11), A116 CrossRef CAS PubMed.
- Y. F. Yuan, J. P. Tu, H. M. Wu, C. Q. Zhang, S. F. Wang and X. B. Zhao, J. Power Sources, 2007, 165(2), 905 CrossRef CAS PubMed.
- X. Xie, Z. Yang, Z. Feng and J. Huang, Electrochim. Acta, 2014, 149, 101 CrossRef CAS PubMed.
- Z. Wang, D. Luan, S. Madhavi, Y. Hu and X. W. Lou, Energy Environ. Sci., 2012, 5(1), 5252 CAS.
- J. Z. Wu, J. P. Tu, Y. F. Yuan, M. Ma, X. L. Wang, L. Zhang, R. L. Li and J. Zhang, J. Alloys Compd., 2009, 479(1–2), 624 CrossRef CAS PubMed.
- R. Wang, Z. Yang, B. Yang, X. Fan and T. Wang, J. Power Sources, 2014, 246, 313 CrossRef CAS PubMed.
- S. H. Lee, C. W. Yi and K. Kim, J. Phys. Chem. C, 2011, 115(5), 2572 CAS.
- Z. Zhang, Z. H. Yang, R. J. Wang, Z. B. Feng, X. Xie and Q. F. Liao, Electrochim. Acta, 2014, 134, 287 CrossRef CAS PubMed.
- J. Vatsalarani, S. Geetha, D. C. Trivedi and P. C. Warrier, J. Power Sources, 2006, 158(2), 1484 CrossRef CAS PubMed.
- J. Vatsalarani, D. C. Trivedi, K. Ragavendran and P. C. Warrier, J. Electrochem. Soc., 2005, 152(10), A1974 CrossRef CAS PubMed.
- H. M. Xiao, W. D. Zhang, M. X. Wan and S. Y. Fu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47(17), 4446 CrossRef CAS PubMed.
- L. Geng, Y. Zhao, X. Huang, S. Wang, S. Zhang, W. Huang and S. Wu, Synth. Met., 2006, 156(16–17), 1078 CrossRef CAS PubMed.
- H. C. Kang and K. E. Gecheler, Polymer, 2000, 41, 6931 CrossRef CAS.
- Y. Wang, W. Jia, T. Strout, A. Schempf, H. Zhang, B. Li, J. Cui and Y. Lei, Electroanalysis, 2009, 21(12), 1432 CrossRef CAS PubMed.
- A. Batool, F. Kanwal, M. Imran, T. Jamil and S. A. Siddiqi, Synth. Met., 2012, 161(23–24), 2753 CrossRef PubMed.
- J. H. Kim, Y. S. Lee, A. K. Sharma and C. G. Liu, Electrochim. Acta, 2006, 52(4), 1727 CrossRef CAS PubMed.
- Z. Guo, K. Shin, A. B. Karki, D. P. Young, R. B. Kaner and H. Thomas Hahn, J. Nanopart. Res., 2008, 11(6), 1441 CrossRef.
- R. Powers and M. Breiter, J. Electrochem. Soc., 1969, 116, 719–729 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the experimental steps of the synthesis, electrode assembly and tests. See DOI: 10.1039/c5ra01452b |
| This journal is © The Royal Society of Chemistry 2015 |