Mn-doped ZnS quantum dots with a 3-mercaptopropionic acid assembly as a ratiometric fluorescence probe for the determination of curcumin†
Abstract
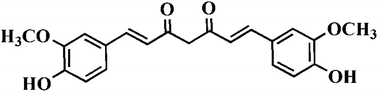
Mn-doped ZnS quantum dots capped with 3-mercaptopropionic acid (MPA) were synthesized by a facile method in aqueous solution as a ratiometric fluorescent (I590 nm/I458 nm) probe for curcumin. Curcumin caused defect-related fluorescence quenching of the probe at 458 nm, whereas the orange transition emission of the probe at 590 nm changed only negligibly with variations in curcumin concentration. The MPA-assembled Mn-doped ZnS quantum dots were used for the simple, sensitive and non-toxic determination of curcumin. Under the optimized conditions, the response to curcumin was linear in the range 0.16–16.9 μM with a detection limit of 37 nM. The method was successfully applied to the determination of trace amounts of curcumin in urine samples with recoveries of 97.73–109.73%.

 Please wait while we load your content...
Please wait while we load your content...