Multi stimuli response of a single surfactant presenting a rich self-assembly behavior†
Abstract
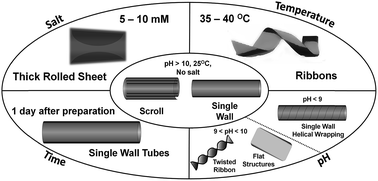
This work reports on the multi stimuli sensitivity of a cholic acid derivative in a buffer. The molecules self-organize in single walled tubules at room temperature and pH 8.0–9.5. By increasing the pH to 10.0–12.0, these tubules open up and form scrolls, which transform by aging into different tubular structures. A further evolution from scrolls or tubules into ribbons is induced by increasing the temperature. Moreover, a transition into rolled lamellae can be triggered by adding NaCl. All transitions are reversible and accompanied by drastic molecular rearrangements, manifested by spectroscopic and imaging techniques. Such rich multi stimuli responsiveness is usually accomplished by block copolymers or peptides and seldom by pure surfactants. The reported surfactant provides in addition an uncommon variety of structures. The sharpness of the spectroscopic response suggests that the system could be employed in physico-chemical sensors with a rather narrow dynamic range.

 Please wait while we load your content...
Please wait while we load your content...