An odorless thia-Michael addition using Bunte salts as thiol surrogates†
Abstract
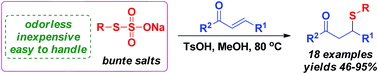
A newly developed C–S bond formation process via acid-catalyzed thia-Michael addition has been demonstrated. The protocol, in which Bunte salts generated from odorless and inexpensive sodium thiosulfate and organic halides are used as the thiol precursors, provides an efficient approach for the synthesis of β-sulfido carbonyl compounds.

 Please wait while we load your content...
Please wait while we load your content...