Novel Bi12ZnO20–Bi2WO6 heterostructures: facile synthesis and excellent visible-light-driven photocatalytic activities†
Abstract
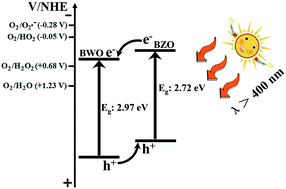
Novel sillenite-type Bi12ZnO20–Bi2WO6 heterostructure photocatalysts were successfully prepared via a controllable partial precipitate conversion strategy, employing previously-prepared Bi2WO6 nanosheets as precursors of Bi3+. The phases and morphologies of the products were characterized by powder X-ray diffraction, energy dispersive spectrometry, X-ray photoelectron spectroscopy, high resolution transmission electron microscopy and scanning electron microscopy. Compared to the sample (Bi12ZnO20 + Bi2WO6) obtained by physical mixing technology and the single-component photocatalyst (Bi12ZnO20 and Bi2WO6), such sillenite-type Bi12ZnO20–Bi2WO6 heterostructures exhibited higher visible-light-driven photocatalytic activity, which could be ascribed to matching band positions and intimate interfacial contact between different components that could benefit the efficient separation of photo-generated carriers.

 Please wait while we load your content...
Please wait while we load your content...