A photoluminescent biosensor based on long-range self-assembled DNA cascades and upconversion nanoparticles for the detection of breast cancer-associated circulating microRNA in serum samples†
Jianming Lana,
Fadi Wenb,
Fangmeng Fuc,
Xi Zhangb,
Shuxian Caib,
Zhijing Liub,
Dongzhi Wub,
Chunyan Li*a,
JingHua Chen*b and
Chuan Wang*c
aDepartment of Basic Chemistry, Faculty of Pharmacy, Fujian Medical University, Fuzhou, Fujian 350108, P. R. China. E-mail: 1135373171@qq.com
bDepartment of Pharmaceutical Analysis, Faculty of Pharmacy, Fujian Medical University, Fuzhou, Fujian 350108, P. R. China. E-mail: cjh_huaxue@126.com; Fax: +86-591-22862016; Tel: +86-591-22862016
cDepartment of Breast Surgery, Fujian Medical University Union Hospital, Fuzhou, Fujian 350001, P. R. China
First published on 5th February 2015
Abstract
A photoluminescent biosensor was developed for the detection of microRNA (miRNA) combined signal amplification of long-range self-assembled DNA cascades with upconversion nanoparticles. The sensor exhibited superior sensitivity with a detection limit of microRNA-21 (miR-21) as low as 1 fM, which may represent the potential of being used in miRNA analysis and clinical diagnosis of breast cancer.
Currently, breast cancer still threatens women's health around the world. However the initial symptoms are not very obvious, meaning that a definite diagnosis is retarded. So it is quite desirable to develop detection techniques that can make a definite diagnosis early. Recent research has found a close link between microRNAs (miRNAs) and breast cancer. MiRNAs are endogenous non-coding RNAs that control gene expression by targeting miRNAs and triggering either translation repression or RNA degradation, and consist of ∼22 nt nucleotides in length.1 Compared to protein markers, abnormal expression of miRNAs (e.g., miR-21) appears earlier, which indicates that miRNAs are more beneficial for early diagnosis and more suitable as tumor markers.2 Therefore, it is necessary to construct sensitive and selective sensing methods for miRNA detection. This would promote basic biomedical research progress and help breast cancer diagnosis.
Now the detection techniques for miRNA mainly include capillary electrophoresis,3 microarray-based detection,4 northern blotting,5 electrochemical biosensors,6 and fluorescent biosensors.7 Among all these techniques, fluorescent biosensors have been recognized as a promising tool for miRNA assay due to their good sensitivity and selectivity, fast analysis, low cost, easy-to-perform, remote control and biocompatibility. However, due to the low concentration of miR-21 and the complex background of serum, conventional fluorescent biosensors do not satisfy the demands of clinical diagnosis for miR-21 detection in serum. To increase analytical sensitivity, many signal amplification techniques have been reported recently.8 Specifically, many isothermally exponential signal amplification strategies based on enzyme assisted target recycling were developed for highly sensitive and selective detection of miRNA under a constant temperature within minutes.9 Nevertheless, such signal amplifications require a specific enzyme recognition process, which hampers the further use. To overcome the shortcomings of the above methods, many long-range self-assembled DNA nanostructures have been fabricated10 and applied in biosensing,11 using long-range self-assembled DNA nanostructures as a carrier for signal amplification, without the aid of any enzyme, or sophisticated equipment. Importantly, this newly proposed biosensor can detect ultra-low concentration of the target DNA even in cell lysates or human serum, with sensitivity similar to that of polymerase chain reaction (PCR). Furthermore, this biosensor based on DNA nanostructures is more biocompatible, more hydrophilic, and thus less prone to nonspecific adsorption onto the substrate surface. Both of these may contribute to the amazing application in the area of clinical miRNAs analysis and cancer diagnostics of the biosensor.
Nowadays, as special photoluminescent probe, lanthanide-doped upconversion nanoparticles (UCNPs) have thus received considerable attention on their synthesis and bioassay. Compared to organic dyes,12 fluorescent proteins and quantum dots (QDs),13 UCNPs can be excited by near infrared reflection (NIR) light which allows for tissue penetration to a depth of centimetres14 and avoids autofluorescence from the biological tissue,15 leading to improved detection sensitivity. In addition, UCNPs have the properties of tunable multicolor emission, high chemical stability, large anti-Stokes shifts, and low in vitro and in vivo toxicity.16 To date, hexagonal (β-) phase NaYF4 co-doped with Yb3+/Er3+ is regarded as the most effective infrared-to-visible upconversion (UC) phosphor,17 as the low maximal optical phonon energy in NaYF4 suppresses nonradioactive multiphonon relaxation processes, which can be responsible for a considerable reduction of the emission intensity.18 However, the unmodified UCNPs present hydrophobic, which is unfavorable for the biocompatibility. Therefore, surface modification is significant to make them biocompatible and provide reactive groups for subsequent bioconjugation to various biomolecules for enhanced circulation and targeting.
Spurred on by all above findings, herein we designed a novel photoluminescent biosensor combined signal amplification of self-assembled DNA cascades with UCNPs, which can be used for the detection of miRNA in breast cancer patients' serum. We adopted thermal decomposition synthesis19 to produce β-NaYF4:Yb3+/Er3+ UCNPs with surfaces presenting carboxyl groups, which were introduced by coating dimercaptosuccinic acid (DMSA) on them. The as-resulted UCNPs were applied in photoluminescent biosensor, which would solve the interference of biological substrates (e.g., protein) in practical sample and well improve miR-21 detection sensitivity.
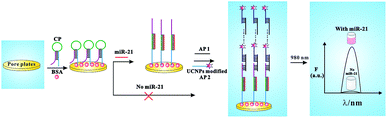
According to the signal amplification based on self-assembled DNA cascades, the sensing strategy was proposed as shown in Fig. 1. Firstly, the hairpin capture probes (CP) are assembled on the surface of 96 pore plates. When adding a target miR-21 (T1) that hybridized with green sequence of CP, the hairpin probes will be opened and transform into flexible short linear structure along with purple sequences of CP released. When adding auxiliary probe1 (AP1) and auxiliary probe2 (AP2), CP can begin a process of cascade hybridization by capturing AP1 and AP2, because the purple sequences of CP can be partly complementary with AP1, and DNA cascades can be self-assemble by AP1 and AP2. Finally, the long-range self-assembled DNA nanostructures formed on the surface of 96 pore plates. Because NaYF4:Yb3+/Er3+ UCNPs are modified on AP2, the DNA nanostructures generate a remarkable amplified UC photoluminescent signal excited by the light of 980 nm. Whereas, the DNA nanostructures self-assembled by AP1 and AP2 can't be coupled on the surface of 96 pore plates due to the closure of purple sequences of CP, when the mutation sequence exists or T1 does not exist. Thus only weak fluorescence signal is observed due to a small amount of nonspecific adsorption. So, the ultra high sensitive detection of T1 can be realized by detecting the change of UC photoluminescent signal in the presence or absence of T1.
In this work, the NaYF4:Yb3+/Er3+ UCNPs were synthesized by thermal decomposition of rare-earth stearates as precursors in OA–ODE system. The as-prepared UCNPs were characterized by XRD and TEM. The results show that the UCNPs are of hexagonal phase (β) and high crystallinity, and appear fine monodispersity, uniformly spherical shape with an average size of about 25 nm (as shown in Fig. 2a–e). After bioconjugation, the UCNPs could be assembled to the pore plate via the DNA cascades. The more the NaYF4:Yb3+/Er3+ UCNPs assembling to the pore plate are, the greater the UC photoluminescent signal is. According to the result of fluorescence spectroscopy as shown in Fig. 2f, the emission peak at 657 nm was chosen as the testing signal, which is the strongest peak and is assigned to the 4F9/2 → 4I15/2 transition of Er3+ ions.
The photoluminescence measurements were performed to serve as a proof of concept to test the principle of our design. In the presence or absence of only target miR-21, nearly no UC photoluminescent signal was observed (Fig. 3a and b), due to no NaYF4:Yb3+/Er3+ UCNPs assembled to the pore plate. Although AP1 and AP2 can self-assemble to be DNA cascades, the DNA cascades cannot link to the plate electrode surface without target miRNA-21, thus only weak photoluminescent signal was observed (Fig. 3c), resulting from nonspecific adsorption. In the presence of target miR-21, if there was only AP1 and no AP2 in the solution, still only a weak photoluminescent signal was observed (Fig. 3d). However, when the solution containing AP1 and AP2 was dripped on the surface of the pore plate, the DNA cascades were self-assembled by numerous AP1 and AP2 immobilized firmly on the pore plate via target miR-21 and CP. Thus the large number of NaYF4:Yb3+/Er3+ UCNPs can be assembled to the pore plate, producing a remarkable amplified UC photoluminescent signal (Fig. 3e). All above photoluminescent results verify the validity of the sensing strategy as we designed.
Fig. 4 shows the photoluminescence detection of target miRNA under the optimum conditions. It can be found that the photoluminescence intensities increase with the concentrations of target miR-21 from 10 fM to 1 pM. And the photoluminescence intensity is linearly dependent on the logarithm (log) of concentration of target miR-21. The correlation equation can be expressed as F = 310.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(cmiRNA) − 310.7 (R = 0.9945), where F is the photoluminescence intensities at 657 nm in the presence of miR-21. According to calculation, the limit of detection (LOD) based on 3σ method is 1 fM. A series of five repetitive measurements with 100 fM miR-21 were used to investigate the precision of the proposed method, and then we obtained a relative standard deviation (RSD) of 2.91%, demonstrating good reproducibility of the assay.
log(cmiRNA) − 310.7 (R = 0.9945), where F is the photoluminescence intensities at 657 nm in the presence of miR-21. According to calculation, the limit of detection (LOD) based on 3σ method is 1 fM. A series of five repetitive measurements with 100 fM miR-21 were used to investigate the precision of the proposed method, and then we obtained a relative standard deviation (RSD) of 2.91%, demonstrating good reproducibility of the assay.
In order to further confirm that the higher sensitivity of the proposed photoluminescent biosensor was partially ascribed to the UCNPs, an analogic labeled fluorescent sensor without the UCNPs was designed for miR-21 detection. In such a fluorescent sensor, the AP2 was labeled with a fluorophore (FAM) at the 3′ end. Upon the addition of miR-21, it could hybridize with CP to open the hairpin structure coupling with a cascade hybridization by capturing AP1 and AP2. Owing to the photophysical properties of FAM, the solution displayed fluorescence emission at 520 nm upon the excitation at 495 nm. As shown in Fig. 5, the fluorescence intensity enhancement increased linearly with the increase of miR-21 concentration in the range from 100 fM to 1 pM and the LOD was calculated to be 80 fM. However, this method suffered from high background signal, because the signal-generating fluorophore moiety was held even in the absence of miR-21 (as shown in curve (a)). Compared with the above method, our proposed photoluminescence based on UCNPs has higher sensitivity due to the photostability of UCNPs.
Compared to the above-described methods, the proposed method has more advantages. First, our proposed photoluminescent biosensor used long-range self-assembled DNA nanostructures as a carrier for signal amplification. So, each copy of the target can act as a trigger to connect a DNA nanostructure to a capture probe on the electrode surface. Then, a great amount of UCNPs can be bound to the DNA nanostructures, which eventually result in significantly amplified photoluminescent signals. Second, this biosensor based on DNA nanostructures is more biocompatible, more hydrophilic, and thus less prone to nonspecific adsorption onto the substrate surface, which may well reduce the background signal. Third, the probe DNA molecules self-assembled onto the surface of the particles should be presented in a multivalent manner, which might increase the affinity with the analyte. Both of these advantages may contribute to the amazing application in the area of clinical miR-21 analysis and breast cancer diagnostics.
To examine the selectivity of our proposed amplified biosensor, we performed a comparison study on mismatch targets and perfect complementary target. Fig. S5 in ESI† shows the photoluminescence intensities for the different kinds of targets, including the perfect complementary target miR-21 with concentration of 1 pM and single-base mismatch target (1 MT), two-base mis-match target (2 MT), and non-complementary (NC) sequence, each with a concentration of 1 nM. As expected, single-base mismatch target results in 8.9% increasing of the photoluminescence intensity (57.7 a.u.) compared to blank treatment (0%, 0.1 a.u.) and perfect complementary target miR-21 (100%, 648.6 a.u.). Additionally, using the two-base mismatch target causes 5.2% increasing of the photoluminescence intensity (33.5 a.u.), whereas the non-complementary sequence leads to only 2.0% increasing of the photoluminescence intensity (13.0 a.u.). These results demonstrate that our proposed biosensor shows high selectivity for the perfect complementary target miR-21.
In reality, the method should be able to distinguish the target miRNA from other miRNAs. Thus we selected the target miR-21 and two other miRNAs (miR-155 and miR-16) as study objects to evaluate the effectiveness of an affinity assay in analyte detection by similar assay procedure. As shown in ESI, Fig. S6,† both miR-155 and miR-16 generate insignificant photoluminescence changes compared to the perfect match target miR-21. The photoluminescence intensity of miR-21 is approximately 30-fold higher than that of miR-155 and miR-16. These results suggest that the proposed method exhibits high sequence specificity in actual testing. In addition, the stability of the biosensor was also investigated. The modified pore plate still could be used to detect target miR-21 without significant decreasing of photoluminescence intensity, when it was immersed in buffer solution and stored at 4 °C for 24 h.
To determine whether the developed photoluminescent biosensor could be applied for analyzing concentrations of miR-21 in real clinical samples, the serum samples from 10 newly diagnosed breast cancer patients and 10 healthy donors were tested by the proposed photoluminescent biosensor and a commercial qRT-PCR kit as reference. Written informed consent was obtained from all participants via an institutional consent form. The study and this consent procedure were approved by the ethics committee of Fujian Medical University Union Hospital (Ethical certification no. E2014021). As shown in Fig. 6, the photoluminescence intensities of the serum from breast cancer patients was about 2.2 times higher than that obtained from the healthy donors serum, suggesting an up-regulation of miR-21 expression in the breast cancer patients serum. This result was in good agreement with reported literatures.20 Furthermore, the designed DNA sensor was applied for the detection of miR-21 in human serum samples by using standard addition method. We chose cancer patient serum sample 1 as an example. A series of synthetic miR-21 were spiked into serum sample 1, respectively, in equal volumes to establish a calibration curve. The concentration of miR-21 in the original serum sample 1 was calculated to be 4.2 fM (as shown in ESI, Fig. S7†). Using the same method, the concentrations of miR-21 in the other nine serum samples were also detected and calculated to be 18.2, 22.3, 34.5, 45.2, 37.4, 28.6, 32.6, 47.1, and 14.9 fM, respectively. The qRT-PCR analysis of miR-21 in the same samples identified that the results obtained from the two methods are basically the same considering the experimental errors. So, the proposed photoluminescent biosensor holds promise in practical application with great accuracy and reliability for miRNA detection.
In summary, we have designed a photoluminescent biosensor combined signal amplification of self-assembled DNA cascades with NaYF4:Yb3+/Er3+ UCNPs, which can be used for the detection of miRNA in actual sample. The use of UCNPs in assays provides the unique nature of UC photoluminescence, without interference of background fluorescence from the carrier fluid or the assay biochemistry, significantly decreasing the LOD that is unavailable from conventional assays. And the optical properties of UCNPs are inert to the environment (e.g., buffer chemistry and assay temperature).21 So, the detection process is unaffected by the sampled fluid and is stable under a large variety of environmental sampling conditions. Using long-range self-assembled DNA nanostructures as a carrier for signal amplification is more biocompatible and thus less prone to nonspecific adsorption without complicated process. The results show that the proposed biosensor has the advanced performances, such as fast detecting, high precision, good reliability, high sequence specificity and low LOD of 1 fM. Therefore, the proposed biosensor can provide a simple and convenient method to quantitative detection of miRNA in real samples.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (21375017, 21205015, 21105012), the National Science Foundation for Distinguished Young Scholars of Fujian Province (2013J06003), the Key Project of Fujian Science and Technology (2013Y0045), Program for Fujian University Outstanding Youth Scientific Research (JA11105, JA10295), Program for New Century Excellent Talents of Colleges and Universities in Fujian Province (JA13130), the Foundation of Fuzhou Science and Technology Bureau (2013-S-122-4), the Medical Elite Cultivation Program of Fujian, P.R.C (2014-ZQN-ZD-26).Notes and references
- D. P. Bartel, Cell, 2004, 116, 281 CrossRef CAS.
- (a) T. Hampton, JAMA, J. Am. Med. Assoc., 2007, 297, 937 CrossRef CAS PubMed; (b) L. Zhang and G. Coukos, Cell Cycle, 2006, 5, 2216 CrossRef CAS.
- N. Khan, J. Cheng, J. P. Pezacki and M. V. Berezovski, Anal. Chem., 2011, 83, 6196 CrossRef CAS PubMed.
- J. M. Thomson, J. Parker, C. M. Perou and S. M. Hammond, Nat. Methods, 2004, 1, 47 CrossRef CAS PubMed.
- A. Valoczi, C. Hornyik, N. Varga, J. Burgyan, S. Kauppinen and Z. Havelda, Nucleic Acids Res., 2004, 32, e175 CrossRef PubMed.
- Z. Q. Gao, H. M. Deng, W. Shen and Y. Q. Ren, Anal. Chem., 2013, 85, 1624 CrossRef CAS PubMed.
- C. Chen, D. A. Ridzon, A. J. Broomer, Z. Zhou, D. H. Lee, J. T. Nguyen, M. Barbisin, N. L. Xu, V. R. Mahuvakar, M. R. Andersen, K. Q. Lao, K. J. Livak and K. J. Guegler, Nucleic Acids Res., 2005, 33, e179 CrossRef PubMed.
- (a) C. Chen, D. A. Ridzon, A. J. Broomer, Z. Zhou, D. H. Lee, J. T. Nguyen, M. Barbisin, N. L. Xu, V. R. Mahuvakar, M. R. Andersen, K. Lao, Q. Livak and K. J. Guegler, Nucleic Acids Res., 2005, 33, e179 CrossRef PubMed; (b) A. Valoczi, C. Hornyik, N. Varga, J. Burgyan, S. Kauppinen and Z. Havelda, Nucleic Acids Res., 2004, 32, e175 CrossRef PubMed.
- (a) B. C. Yin, Y. Q. Liu and B. C. Ye, J. Am. Chem. Soc., 2012, 134, 5064 CrossRef CAS PubMed; (b) Q. Xi, D. M. Zhou, Y. Y. Kan, J. Ge, J. K. Wu, R. Q. Yu and J. H. Jiang, Anal. Chem., 2014, 86, 1361 CrossRef CAS PubMed; (c) F. Degliangeli, P. Kshirsagar, V. Brunetti, P. P. Pompa and R. Fiammengo, J. Am. Chem. Soc., 2014, 136, 2264 CrossRef CAS PubMed.
- (a) N. C. Seeman, Trends Biochem. Sci., 2005, 30, 119 CrossRef CAS PubMed; (b) C. Teller and I. Willner, Trends Biotechnol., 2010, 28, 619 CrossRef CAS PubMed; (c) A. V. Pinheiro, D. R. Han, W. M. Shih and H. Yan, Nat. Nanotechnol., 2011, 6, 763 CrossRef CAS PubMed; (d) D. S. Liu, E. J. Cheng and Z. Q. Yang, NPG Asia Mater., 2011, 3, 109 CrossRef.
- J. Huang, Y. R. Wu, Y. Chen, Z. Zhu, X. H. Yang, C. Y. Yang, K. M. Wang and W. H. Tan, Angew. Chem., Int. Ed., 2011, 50, 401 CrossRef CAS PubMed.
- X. P. He, R. H. Li, S. Maisonneuve, Y. B. Ruan, G. R. Chen and J. Xie, Chem. Commun., 2014, 50, 14141 RSC.
- W. Ma, H. T. Liu, X. P. He, Y. Zang, J. Li, G. R. Chen, H. Tian and Y. T. Long, Anal. Chem., 2014, 86, 5502 CrossRef CAS PubMed.
- (a) A. M. Smith, M. C. Mancini and S. M. Nie, Nat. Nanotechnol., 2009, 4, 710 CrossRef CAS PubMed; (b) X. H. Gao, Y. Y. Cui, R. M. Levenson, L. W. K. Chung and S. M. Nie, Nat. Biotechnol., 2004, 22, 969 CrossRef CAS PubMed.
- G. Y. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Ägren and P. N. Prasad, ACS Nano, 2010, 4, 3163 CrossRef CAS PubMed.
- (a) S. Heer, O. Lehmann, M. Haase and H. U. Güdel, Angew. Chem., Int. Ed., 2003, 42, 3179 CrossRef CAS PubMed; (b) X. Wang and Y. D. Li, Chem. Commun., 2007, 2901 RSC.
- K. W. Krämer, D. Biner, G. Frei, H. U. Güdel, M. P. Hehlen and S. R. Lüthi, Chem. Mater., 2004, 16, 1244 CrossRef.
- L. Y. Wang and Y. D. Li, Chem. Mater., 2007, 19, 727 CrossRef CAS.
- Q. Q. Zhan, J. Qian, H. J. Liang, G. Somesfalean, D. Wang, S. L. He, Z. G. Zhang and S. Andersson-Engels, ACS Nano, 2011, 5, 3744 CrossRef CAS PubMed.
- (a) S. Asaga, C. Kuo, T. Nguyen, M. Terpenning, A. E. Giuliano and D. S. B. Hoon, Clin. Chem., 2011, 57, 84 CrossRef CAS PubMed; (b) F. J. Wang, Z. G. Zheng, J. F. Guo and X. F. Ding, Gynecol. Oncol., 2010, 119, 586 CrossRef CAS PubMed.
- G. Y. Chen, H. L. Qiu, P. N. Prasad and X. Y. Chen, Chem. Rev., 2014, 114, 5188 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, XRD, IR, TEM, fluorescence spectrum and Table S1. See DOI: 10.1039/c5ra01288k |
| This journal is © The Royal Society of Chemistry 2015 |