Rhamnolipid biosurfactant adsorption on a plasma-treated polypropylene surface to induce antimicrobial and antiadhesive properties†
Hamidreza Hajfarajollah‡
a,
Saeid Mehvari‡a,
Mahmoud Habibian*a,
Babak Mokhtarania and
Kambiz Akbari Noghabib
aChemistry and Chemical Engineering Research Center of Iran, P. O. Box 14335-186, Tehran, Iran. E-mail: mhabibian@ccerci.ac.ir; Fax: +98 2144787781; Tel: +98 2144787784
bNational Institute of Genetic Engineering and Biotechnology, P. O. Box 14155-6343, Tehran, Iran
First published on 25th March 2015
Abstract
A glycolipid type of biosurfactant (rhamnolipid), which is obtained from Pseudomonas aeruginosa MA01, was adsorbed on a polypropylene film to produce an antimicrobial and antiadhesive polymeric surface for the first time. The polypropylene film was modified using oxygen and air plasma. The effects of the plasma operating conditions, including the plasma power and the time of plasma exposure, were studied. The characteristics and hydrophobicity of the polypropylene surface were evaluated by several techniques including ATR-FTIR, XPS, SEM and AFM as well as measuring water contact angles (WCA). The results confirmed the successful attachment of rhamnolipid on plasma-treated surfaces, however in different degrees based on the plasma conditions. The antibacterial and antiadhesive performance of the rhamnolipid-adsorbed-films was investigated against pathogenic bacteria, and the results showed considerable activity of the surface to reduce the number of bacteria on the treated polymeric film. The optimum plasma conditions, in which the best antimicrobial and antiadhesive surface was obtained, were revealed as a power of 50 W and an exposure time of 6 min with air as the plasma gas.
Introduction
The safety and quality of ready-to-eat “fresh” food products is one of the major new challenges in the food industries.1 The development of packaging materials with antibacterial or antiadhesive properties and ensuring their hygienic status remains a fundamental scientific, technological and industrial challenge.2 Polymers are the most frequently used materials in the food industries, especially polypropylene (PP), which is often used in soft or hard packaging. Antibacterial/antiadhesive packaging materials can effectively inhibit the growth or prevent the adhesion of microorganisms on the surface of the packing materials. L. monocytogenes is an important foodborne pathogen, which can cause the serious illness, listeriosis. Furthermore, S. aureus is the bacterium, which causes staph infections, and a Gram negative bacterium, K. pneumoniae, is the bacterium responsible for pneumonia.3 These bacteria have been found in a wide variety of food products such as raw vegetables, raw meat, dairy products and ready-to-eat foods.4In recent years, many attempts have been made to functionalize surfaces with chemical antimicrobial agents to manufacture antimicrobial films.5,6 However, very few studies have been reported on the use of natural antimicrobial agents produced by microorganisms. Surface modification has been performed using natural biological substances like bacteriocins, which possess antimicrobial activity.7 Nisin is currently the only bacteriocin widely used as a food preservative.7 This peptide, which is produced by Lactococcus lactis subsp. lactis, exerts rapid bactericidal effects against a broad spectrum of Gram-positive bacteria and food pathogens, including L. monocytogenes, S. aureus, B. cereus, and C. botulinum.8,9 Karam et al.1 investigated nisin adsorption on polyethylene surfaces that were previously modified using argon/oxygen (Ar/O2) plasma, nitrogen (N2) plasma and plasma-induced grafting of acrylic acid. Maximum antibacterial activity was recorded on the Ar/O2 plasma followed by acrylic acid and N2 treated films, and the lowest activity was observed on the native film.
Biosurfactants are amphipathic compounds excreted by microorganisms showing surface activity.10 The rhamnolipid biosurfactant produced by Pseudomonas aeruginosa is a glycolipid composed of one or two rhamnose molecules linked to one or two fatty acid alkyl chains. They are synthesized as a mixture of homologs mainly composed of di-rhamnolipids and mono-rhamnolipids.11 Rhamnolipid biosurfactants show several properties, such as surface activity, emulsification, better environmental compatibility, biodegradability and specific activity, at extreme temperatures, pH and salinity.12 These properties are very useful in the processing industries.10 The rhamnolipid biosurfactant has demonstrated great antiadhesive and antimicrobial activity against several microorganisms such as Gram-positive bacteria (S. aureus, B. subtilis, C. perfringens) Gram-negative bacteria (C. perfringens, E. coli, E. aerogenes) and fungi (P. infestans, P. capsici, B. cinerea, F. graminearum and Mucor spp).4 Because of their antimicrobial activity, biosurfactants are used as food preservatives.13 With regard to the above mentioned explanation, it can be a noble idea to employ biosurfactants as antimicrobial agents for active packaging.
The objective of this study is to evaluate the use of plasma treatment to modify a polymeric surface with the goal of rhamnolipid adsorption on the surface. Polypropylene, a well-known polymer in the food and biomedical sectors, was subjected to a plasma environment in different conditions. The rhamnolipid biosurfactant was then adsorbed on the plasma-treated surface. The treated polymeric surfaces were characterized by different methods before and after the treatment. Finally, the antimicrobial and antiadhesive activities of the plasma-treated polymeric surface were evaluated.
Results and discussion
In order to simplify and clarify the real parameters affecting the surfaces, primary experiments were performed to find out the optimum exposure time. This optimum time was selected for further tests.Primary experiments for plasma exposure time optimization
The untreated PP film has hydrophobic properties and its water contact angle (WCA) was 91.3°. Plasma exposure times of 2, 4, 6, 8 and 10 minutes, using air and oxygen gases and a power of 50 W, were studied and the measured water contact angles (WCAs) in the case of air gas were 70.7, 66.1, 51.2, 50 and 50.5°, respectively. The most significant decrease in hydrophilicity appeared in the exposure time of 6 min and further extension of time did not change the WCA. A similar trend was observed using oxygen gas. Therefore, the exposure time of 6 minutes was used as the optimum plasma exposure time for further experiments.Hydrophilicity and antimicrobial/antiadhesive activity of the surfaces
Experiments were carried out according to Table 1 with two types of gas (air and oxygen) and four different plasma RF powers (25, 50, 75 and 100 W) at a fixed exposure time of 6 min. The measured contact angles of the PP films after plasma treatment and also after rhamnolipid adsorption have been presented in Table 1. The contact angles of PP surfaces dropped from the original value of 91.3 ± 2.3° to 50.6 ± 1.7° for oxygen treatment with a power of 75 W (PP-O2-75) and to 50.2 ± 2.1 for air treatment with a power of 50 W (PP-Air-50). After rhamnolipid adsorption on the surface, the WCA dropped significantly. The WCA decreased from 50.6 ± 1.7 to 15.9 ± 1.7 for PP-O2-75 and from 50.2 ± 2.1 to 12.2 ± 1.4 for PP-Air-50. The same trend was observed for the remaining samples. The WCA results confirmed that the plasma treatment was effective in improving the surface hydrophilicity. Moreover, the influence of rhamnolipid adsorption on the improvement of the surface hydrophilicity was deeper than only with plasma treatment. The decrease in the contact angle is likely due to the presence of polar groups such as oxygen-containing functional groups.13,14 Fig. 1 illustrates the selected images of water droplets for WCA measurement. The shape of the droplet on the PP surface changes after plasma treatment and more significantly after rhamnolipid adsorption.| Sample name | Plasma gas | Power (W) | Time (min) | WCA (Ɵw) after plasma | WCA (Ɵw) after adsorption | Area of inhibition zoneb (mm2) | |||
|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | K. pneumoniae | P. aeruginosa | ||||||
| a WCA of untreated PP is 91.3°.b Area of untreated film is 15 × 15 = 225 mm2, which shows no inhibition. | |||||||||
| Untreated PP | — | — | — | a | 80.1 ± 3.3 | 225 | 225 | 225 | 225 |
| PP-O2-25 | O2 | 25 | 6 | 68.3 ± 2.5 | 50.1 ± 2.5 | 326.61 | 331.24 | 225 | 225 |
| PP-O2-50 | O2 | 50 | 6 | 57.9 ± 2.1 | 33.3 ± 2.1 | 380.25 | 420.25 | 225 | 225 |
| PP-O2-75 | O2 | 75 | 6 | 50.6 ± 1.7 | 15.9 ± 1.7 | 595.36 | 529 | 225 | 225 |
| PP-O2-100 | O2 | 100 | 6 | 55.9 ± 1.1 | 17.8 ± 1.1 | 580.81 | 538.24 | 225 | 225 |
| PP-Air-25 | Air | 25 | 6 | 60.2 ± 3.3 | 27.8 ± 3.3 | 542.89 | 316.84 | 225 | 225 |
| PP-Air-50 | Air | 50 | 6 | 50.2 ± 2.1 | 12.2 ± 1.4 | 681.21 | 501.76 | 225 | 225 |
| PP-Air-75 | Air | 75 | 6 | 51.1 ± 2.1 | 14.1 ± 2.1 | 630.01 | 475.24 | 225 | 225 |
| PP-Air-100 | Air | 100 | 6 | 51.2 ± 1.6 | 13.8 ± 1.6 | 650.25 | 484 | 225 | 225 |
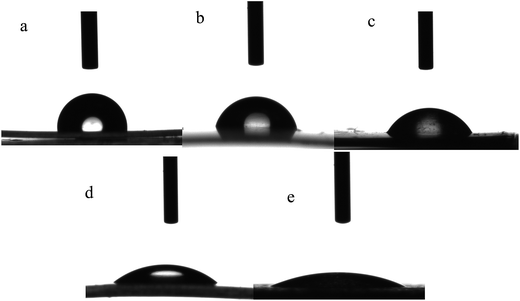 | ||
| Fig. 1 Contact angle images of deionized water droplets over the film surfaces: (a) untreated PP, (b) PP-O2-75 (c) PP-Air-50 (d) PP-O2-75 after adsorption (e) PP-Air-50 after adsorption. | ||
Antimicrobial assays were performed after adsorption of the rhamnolipid on the PP surfaces. The results were compared with the antimicrobial activity of untreated PP and reported as the area (mm2) of inhibition zone. Two Gram-positive (i.e. S. aureus and B. subtilis) and two Gram-negative (i.e. P. aeruginosa and K. pneumonia) bacteria were used for this assay. Fig. S1† shows a schematic illustration of the test.
Table 1 shows that no inhibition zone was formed around the PP films placed on Gram negative bacteria. This is because of the interaction of antimicrobial agents with the cell membrane of bacteria. The outer membrane of Gram negative bacteria may serve as a barrier to the entry of antimicrobial molecules. Hence, the difference between the structure of the cell membrane of Gram positive and Gram negative bacteria is the main reason for the differences in their susceptibilities towards antimicrobial agents.15,16
With a low RF power of 25 W (Table 1), a lower antimicrobial activity of the film was observed. In general, PP-O2-75 and PP-Air-50 samples with 595 and 681 mm2 clear zones, respectively, resulted in the best antimicrobial activities. This can be explained on the basis of rhamnolipid immobilization on the surface. This indicates that the rhamnolipid biosurfactant had immobilized more efficiently through hydrophilic interactions or hydrogen bonds on the samples' surfaces. In addition, the antimicrobial agents are well oriented on the surface to inactivate Gram positive bacteria deposited over them. Comparatively, the air plasma treatment (PP-Air-50) resulted in better final antimicrobial activity than oxygen-treated plasma (PP-O2-75).
The rhamnolipid biosurfactants have a great antiadhesive activity. This activity may help the rhamnolipid-adsorbed-surface to prevent bacterial adhesion. The histograms shown in Fig. S2 of the ESI† data present the antiadhesive activity of the rhamnolipid-adsorbed-films. The results showed that almost all the treated samples could prevent the bacterial adhesion of both Gram positive and negative bacteria on the surface. However, the number of Gram negative K. pneumoniae adhered onto the films was generally higher than that of B. subtilis, which may be due to the difference between the physicochemical characteristics of the bacteria and material.17 The antibacterial samples did not fully inhibit the formation of the bacterial biofilm after 18 h incubation, which is in agreement with Zhang et al.18 However, over 60% inhibition is observed for some samples, suggesting the capability of rhamnolipid to inhibit bacterial adhesion.
Surface topology and morphology analysis
Fig. 2 shows the two-dimensional and three-dimensional AFM surface morphologies of the films before and after plasma treatment. Table 2 also shows the values for the most common surface parameters for the plasma-treated samples before rhamnolipid adsorption. These parameters include average roughness (Ra), root mean roughness (Rq), Skewness (Rsk) and Kurtosis (Rku).19,20 The untreated PP film is quite smooth, with an average roughness (Ra) value of 9.92 ± 1.13 nm and root mean roughness (Rq) value of 10.01 ± 1.33 nm. Experimental evidence has shown that the surface morphology of the polymer films turned rougher after plasma treatment.21 In some cases, however, the plasma-treated surface turned smoother.2 In this study, Ra increased almost for all the treated samples except for the PP-O2-25 sample. In the case of PP-O2-25, the Ra showed a slight decrease from 9.92 to 9.11 nm. In addition, for PP-Air-25, the Ra showed a slight increase from 9.92 to 11.09 nm. It can be noted that with a low power, no appreciable change in roughness occurs. The Ra values for PP-O2-75 and PP-Air-50 increased more than two and three folds, respectively. It is hard to draw a direct conclusion between the roughness parameters and rhamnolipid adsorption to the surface; however, rougher surfaces have generally led to a better rhamnolipid adsorption (so better antimicrobial performance).| Sample | Roughness, Ra (nm) | Root mean squared roughness, Rq (nm) | Roughness skew, Rsk (nm) | Roughness kurtosis, Rku |
|---|---|---|---|---|
| Untreated PP | 9.92 ± 1.13 | 10.01 ± 1.33 | 0.11 ± 0.06 | 2.21 ± 0.41 |
| PP-O2-25 | 9.11 ± 0.31 | 10.21 ± 0.67 | 0.21 ± 0.01 | 3.13 ± 0.31 |
| PP-O2-50 | 19.22 ± 1.11 | 23.18 ± 1.19 | 0.14 ± 0.04 | 3.45 ± 0.27 |
| PP-O2-75 | 20.88 ± 3.35 | 24.41 ± 2.21 | −0.04 ± 0.03 | 4.13 ± 0.56 |
| PP-O2-100 | 19.78 ± 3.67 | 25.45 ± 4.01 | 0.05 ± 0.01 | 3.72 ± 0.61 |
| PP-Air-25 | 11.09 ± 2.11 | 13.56 ± 3.13 | 0.25 ± 0.07 | 3.26 ± 0.32 |
| PP-Air-50 | 27.98 ± 1.88 | 31.76 ± 2.17 | 0.11 ± 0.03 | 3.91 ± 0.48 |
| PP-Air-75 | 29.55 ± 4.50 | 32.04 ± 3.45 | 0.15 ± 0.03 | 3.67 ± 0.45 |
| PP-Air-100 | 30.10 ± 3.12 | 38.11 ± 2.12 | −0.09 ± 0.03 | 3.48 ± 0.17 |
The 3D AFM images of air-treated (B, C, D, E) and O2-treated (F, G, H, I) plasmas are presented in Fig. 2. Considerable differences between the formed patterns are observed. “Lay” is the term used to indicate the direction of the dominant pattern of texture on the surface. For samples that have been treated with O2 gas, the lay is in the front-to-back direction. However, in the case of air plasma, an irregular pattern was formed. In general, the surfaces with irregular structures (air plasma films) showed higher amount of antimicrobial activity (Table 1).
This type of structure may provide more anchoring or filling sites for rhamnolipid adsorption1 and further higher antimicrobial activity. A 3D image of the AFM analysis for a representative sample (PP-Air-50) after rhamnolipid adsorption has been presented in Fig. 2J. In this sample, all the peaks have been covered with the antimicrobial material. In fact, it seems that rhamnolipid molecules filled the valleys and caused the surface to be smooth. This observation can boost the hypothesis that valleys may serve as anchoring or filling sites for the adsorption of other molecules.
The SEM images of the sample PP-Air-50 (which showed the best antimicrobial activity) after plasma treatment and after rhamnolipid adsorption, along with the untreated PP, are illustrated in Fig. 3. In Fig. 3A, a relatively smooth and uniform morphology was observed for the untreated film. After undergoing some alterations in the plasma chamber (Fig. 3B), the modification leads to the presence of some diagonal patterning and irregularly shaped surface texture. This topography is beneficial for the subsequent coupling processes due to the surface area and increase in roughness.22 In fact, the generated pattern on the plasma-treated specimen is both due to the ablation and functionalization of the surface, which leads to surface restructuring.23 However, these changes in surface morphology are not appreciable. In addition, the surface after rhamnolipid adsorption has an entirely different structure. As can be seen in Fig. 3C, a granular like structure is formed after rhamnolipid adsorption.
Surface chemistry analysis, ATR-FTIR and XPS
Fig. 4 shows the ATR-FTIR pattern of the untreated PP along with the PP-Air-50 sample after plasma treatment and after rhamnolipid adsorption. In the IR spectra of the untreated PP, special interest is focused on the following absorption peaks: the 973 cm−1 rocking vibration (–CH2–), 997 cm−1 rocking vibration (–CH2–), 1167 cm−1 anti-symmetric deformation (–CH3–), 1455 cm−1 symmetric deformation (–CH2–), 1167 cm−1 symmetric deformation (–CH3–), 1167 cm−1 anti-symmetric deformation (–CH–) and 2917 cm−1 symmetric stretching (–CH3–). However, as can be observed in Fig. 4, upon exposure of the untreated sample to plasma discharge, almost no considerable change was detected in the ATR-FTIR of the PP-Air-50 sample. Only a weak broad peak between 3400 and 3500 cm−1 corresponding to the OH group can be taken into consideration. This is not only because of signals overlapping, but also due to the plasma modification depth being limited solely to the top layers of the surface, which cannot be well evidenced by ATR-FTIR. The crystals used in ATR cells (zinc selenide (ZnSe)) for polymers have a low solubility in water and very high refractive index (≈2.4) and average sampling depth of ≈4 μm.24 This sampling depth exceeds the normal thickness of plasma modified layers on a substrate (<100 nm). Nonetheless, ATR-FTIR is still widely used to provide semi-quantitative information on the chemistry of near-surface regions. | ||
| Fig. 4 FTIR patterns of the polymeric film for untreated PP, PP-Air-50 sample, and PP-Air-50 after rhamnolipid adsorption. | ||
The changes in the ATR-FTIR patterns after the adsorption of antimicrobial rhamnolipid on the PP film are clearly detectable (Fig. 4). The O–H stretching of the free hydroxyl groups of rhamnose rings around 3385–3390 cm−1, the stretching bands of the methylene and terminal methyl groups of the acyl chains between 2850 and 2930 cm−1, the stretching band of the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups at approximately 1744 cm−1, the free –COO– band (free carboxyl group of the second fatty acid) around 1560–1580 cm−1, and the C–O–C vibrations (rhamnose rings) at about 1045 cm−1 are some characteristic peaks of the adsorbed antimicrobial agent on the surface. The FTIR pattern of pure mono-rhamnolipid produced by Pseudomonas aeruginosa MA01 can be observed in Fig. S3B in the ESI† file.
O groups at approximately 1744 cm−1, the free –COO– band (free carboxyl group of the second fatty acid) around 1560–1580 cm−1, and the C–O–C vibrations (rhamnose rings) at about 1045 cm−1 are some characteristic peaks of the adsorbed antimicrobial agent on the surface. The FTIR pattern of pure mono-rhamnolipid produced by Pseudomonas aeruginosa MA01 can be observed in Fig. S3B in the ESI† file.
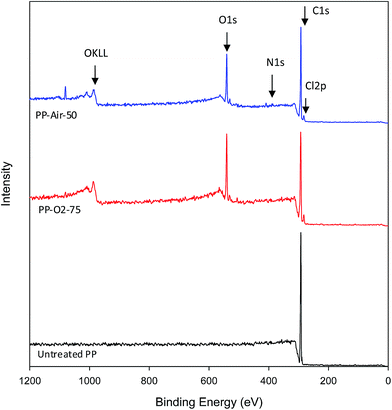
The FTIR pattern cannot tell us in detail about the changes in surface chemistry of the plasma treated film. Therefore, XPS analysis has been performed on the plasma treated PP films, in combination with a labeling technique, for better understanding the functionalities introduced to the polymer surface. Fig. 5 presents the XPS scans of the untreated PP as well as for PP-O2-75 and PP-Air-50 samples with the best antimicrobial activity. The elemental composition, expressed as atomic concentrations of the selected samples, has been presented in Table 2. The scan of the untreated PP shows only one peak, which is attributed to the C1s of the aliphatic carbon bonds or carbon–hydrogen bonds (C–C, C–H) (Fig. 5). It should be noted that the carbon C1s peaks of the untreated sample as well as of PP-O2-75 are shown in Fig. S4 and S5.† The XPS records reveal that no traces of any contaminant element were found on the surface of the untreated PP. After oxygen or air plasma treatment, an increase in oxygen concentration was observed on the films (Table 2 and Fig. 5). This can be associated to the created oxygen functional groups.25 Furthermore, when air was used as a plasma gas, nitrogen element (N1s) was also detected on the surface. Surprisingly, small amount of chlorine (Cl2p) was observed on the surface of PP film for all the plasma treated samples, which may come from the plasma parts as a contaminant.
The amount of carbon was reduced after plasma treatment (Table 3). The decrease of carbon content and the increase of oxygen content on both the samples can be contributed to the introduction of oxygen-containing polar groups (C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –O–C
O, –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –COH) on the surface of polypropylene. The incorporation of oxygen containing polar groups in PP surface may be the main reason for the hydrophilic improvement of PP-O2-75 and PP-Air-50 samples as described in Table 1. In addition, the presence of these oxygenic groups can improve the linkage between the antimicrobial molecules and the surface during the adsorption process.
O, –COH) on the surface of polypropylene. The incorporation of oxygen containing polar groups in PP surface may be the main reason for the hydrophilic improvement of PP-O2-75 and PP-Air-50 samples as described in Table 1. In addition, the presence of these oxygenic groups can improve the linkage between the antimicrobial molecules and the surface during the adsorption process.
| Sample | C | O | N | Cl | O/C | N/C | Cl/C |
|---|---|---|---|---|---|---|---|
| Untreated PP | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| PP-O2-75 | 85.4 ± 0.9 | 13.7 ± 1.1 | 0 | 2.1 ± 0.5 | 0.16 | 0.00 | 0.02 |
| PP-Air-50 | 76.3 ± 1.1 | 15.1 ± 1.2 | 5.6 ± 0.2 | 3.1 ± 0.9 | 0.19 | 0.07 | 0.04 |
Film stability
In order to investigate the stability of the antimicrobial surfaces under the likely application conditions, PP-O2-75 and PP-Air-50 samples were submitted to relatively soft cleaning conditions such as immersion in water for 24 h and in a non-ionic detergent solution. The results showed that both PP-O2-75 and PP-Air-50 samples were stable and maintained their antibacterial activity after contacting water. In case of emerging in a non-ionic detergent solution, the activity of the films reduced by 22% and 13%.Materials and methods
Chemicals and microorganisms
All the chemicals were purchased from Merck (Germany) unless otherwise stated. Pseudomonas aeruginosa MA01, which had been isolated from a spoiled apple in our previous work,11 was used for the production of the rhamnolipid biosurfactant. Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, and Pseudomonas aeruginosa were kindly provided by the University of Tehran and used for antimicrobial or antiadhesive assays.Production, extraction and purification of the rhamnolipid biosurfactant
Pseudomonas aeruginosa MA01 (isolated and identified in the previous work11) was used for rhamnolipid production. This microorganism was pre-cultured in nutrient broth medium at 30 °C and 200 rpm for 14–16 h. 4% inoculation was transferred from this seed culture to the production medium. The medium used for rhamnolipid biosurfactant production includes (g L−1): sunflower oil 20, yeast extract 1, NaNO3 3, MgSO4·7H2O 0.25 and KH2PO4 0.25. After 5–6 days cultivation at 30 °C and 200 rpm, the biosurfactant was extracted from the cell free supernatant using the method of precipitation followed by solvent extraction. The biosurfactant was then purified using column chromatography as explained in the previous work.11 Briefly, a slurry of silica gel 60 in chloroform was poured into a glass column. Crude biosurfactant was dissolved in chloroform and loaded onto the column. Purification was carried out by washing the column with chloroform (to elute neutral lipids), followed by chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol. During the purification steps, analytical TLC (using chloroform
methanol. During the purification steps, analytical TLC (using chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol
methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (65
H2O (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as mobile phase) was used to check the purity of the fractions. The purified biosurfactant was then characterized by TLC, FTIR and ES-MS techniques (Fig. S3†). Finally, the pure mono-rhamnolipid was kept in an appropriate condition for further studies.
2) as mobile phase) was used to check the purity of the fractions. The purified biosurfactant was then characterized by TLC, FTIR and ES-MS techniques (Fig. S3†). Finally, the pure mono-rhamnolipid was kept in an appropriate condition for further studies.
Film preparation
The polypropylene (PP) films, with a thickness of 0.2 mm, were initially cut into 1.5 × 1.5 cm2. The films were then washed with ethanol in an ultrasonic bath to remove possible dust, oily compounds or any other chemicals and wetting agents absorbed on the film surface. The films were dried in an oven at 55 °C for 3 h and stored in a desiccator before use.Plasma treatment
Plasma treatment of the polymeric films was carried out in a plasma chamber (Nano-LF-RF-PC, Diener electronic Technology, Germany) using a RF generator (13.56 MHz, max 100 W). The schematic of the plasma system and plasma equipment are illustrated in Fig. S6 and S7.† The polymeric sample was placed into the chamber (made of quartz glass) and the pressure was reduced to 0.1 mbar by means of a vacuum pump (Trivac, Germany). Process parameters, such as gas type, power and exposure time, were varied to optimize for the best treatment conditions. In order to reduce the number of experiments and thus reducing the costs, the films were initially plasma treated with air and oxygen plasma with a medium power of 50 W at different exposure times to select the optimum exposure time. Because low contact angles correspond to the formation of oxygenic groups, surface hydrophilicity was employed as a response. After this primary experiment, a comprehensive study was performed to see the effect of gas type and power strength on the plasma treatment procedure and finally rhamnolipid adsorption to the surface. Therefore, using oxygen or air plasma, RF powers of 25, 50, 75 and 100 W with the optimized exposure time were studied. It should be noted that the gas flow in all experiments was set to 20 sccm.Adsorption of rhamnolipid on the surface
The plasma treated substrates were immersed into 10 ml of 5 g L−1 biosurfactant solution and left in a shaker incubator (Kühner, Germany) for 16–18 h at 8 °C and 100 rpm. The samples were rinsed with an appropriate buffer (5 times, 5 min with 5 ml of buffer), and then with deionized water (5 times, 5 min with 5 ml of water). Samples were dried and stored at room temperature for further analyses.Surface characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × magnification was used. Samples were stuck with a conducting adhesive on the SEM metallic substrate holder and directly introduced into the chamber. The analytical chamber is equipped by a fully motorized sample manipulator.
000 × magnification was used. Samples were stuck with a conducting adhesive on the SEM metallic substrate holder and directly introduced into the chamber. The analytical chamber is equipped by a fully motorized sample manipulator.Antimicrobial and antiadhesive assays
Conclusion
In this study, we demonstrated the effects of plasma treatment on polypropylene surface properties and then rhamnolipid adsorption on the surface. Both the oxygen and air plasma treatment introduced oxygen-containing polar groups to the surface of PP, and the hydrophilicity of PP was improved. More improvement in hydrophilicity was obtained after rhamnolipid adsorption. The morphology of PP was significantly influenced by the type of plasma treatment conditions as confirmed by AFM and SEM analysis. The overall results of the study confirmed the successful adsorption of rhamnolipid to the surface and consequently appropriate antimicrobial and antiadhesive properties were induced to the surface. The antimicrobial PP film obtained in this study could be a useful choice for usage in food and pharmaceutical industries as packaging materials.References
- L. Karam, C. Jama, A. Mamede, A. Fahs, G. Louarn, P. Dhulster and N. Chihi, React. Funct. Polym., 2013, 73, 1473 CrossRef CAS PubMed.
- F. Poncin-Epaillard, J. M. Herry, P. Marmey, G. Legeay, D. Debarnot and M. N. Bellon-Fontaine, Mater. Sci. Eng., C, 2013, 33, 1152 CrossRef CAS PubMed.
- R. Davis, A. El-Shafei and P. Hauser, Surf. Coat. Technol., 2011, 205, 4791 CrossRef CAS PubMed.
- L. Magalhães and M. Nitschke, Food Control, 2013, 29, 138–142 CrossRef PubMed.
- A. Sadeghnejad, A. Aroujalian, A. Raisi and S. Fazel, Surf. Coat. Technol., 2014, 245(25), 1 CrossRef CAS PubMed.
- A. Asadinezhad, I. Novak, M. Lehocky, V. Sedlarik, A. Vesel, I. Junkar, P. Saha and I. Chodak, Colloids Surf., B, 2010, 77, 246 CrossRef CAS PubMed.
- J. Cleveland, T. J. Montville, I. F. Nes and M. L. Chikindas, Int. J. Food Microbiol., 2001, 71, 1 CrossRef CAS.
- R. W. Jack, J. R. Tagg and B. Ray, Microbiol. Rev., 1995, 59, 171 CAS.
- I. E. Pol and E. J. Smid, Lett. Appl. Microbiol., 1999, 29, 166 CrossRef CAS.
- H. Sharafi, M. Abdoli, H. Hajfarajollah, N. Samie, L. Alidoust, H. Abbasi, J. Fooladi, H. S. Zahiri and K. A. Noghabi, Appl. Biochem. Biotechnol., 2014, 173(5), 1236–1249 CrossRef CAS PubMed.
- H. Abbasi, M. M. Hamedi, T. B. Lotfabad, H. S. Zahiri, H. Sharafi, F. Masoomi, A. A. Moosavi-Movahedi, A. Ortiz, M. Amanlou and K. A. Noghabi, J. Biosci. Bioeng., 2012, 113(2), 211 CrossRef CAS PubMed.
- H. Hajfarajollah, B. Mokhtarani and K. Akbari Noghabi, Appl. Biochem. Biotechnol., 2014, 174(8), 2725–2740 CrossRef CAS PubMed.
- M. Nitschke and S. G. V. A. O. Costa, Trends Food Sci. Technol., 2007, 18, 252 CrossRef CAS PubMed.
- H. Hajfarajollah, B. Mokhtarani, K. Akbari Noghabi, A. Sharifi and M. Mirzaei, RSC Adv., 2014, 4, 42751 RSC.
- D. Duday, C. Vreuls, M. Moreno, G. Frache, N. D. Boscher, G. Zocchi, C. Archambeau, C. Van De Weerdt, J. Martial and P. Choquet, Surf. Coat. Technol., 2013, 218, 152 CrossRef CAS PubMed.
- H. Hajfarajollah, B. Mokhtarani, A. Sharifi, M. Mirzaei and A. Afaghi, RSC Adv., 2014, 4, 13153 RSC.
- Y. H. An and R. J. Friedman, J. Biomed. Mater. Res., 1998, 43, 338 CrossRef CAS.
- W. Zhang, P. K. Chu, J. Ji, Y. Zhang, S. C. Ng and Q. Yan, Biopolymers, 2006, 83, 62 CrossRef CAS PubMed.
- E. S. Gadelmawla, M. M. Koura, T. M. A. Maksoud, M. Elewa and H. H. Soliman, J. Mater. Process. Technol., 2002, 123, 133 CrossRef.
- M. Sedlacek, B. Podgornik and J. Vizintin, Wear, 2009, 266, 482 CrossRef CAS PubMed.
- F. Leroux, C. Campagne, A. Perwuelz and L. Gengembre, J. Colloid Interface Sci., 2008, 328, 412 CrossRef CAS PubMed.
- P. K. Chu, J. Y. Chen, L. P. Wang and N. Huang, Mater. Sci. Eng., R, 2002, 36, 143 CrossRef.
- A. Vesel, I. Junkar, U. Cvelbar, J. Kovac and M. Mozetic, Surf. Interface Anal., 2008, 40, 1444 CrossRef CAS.
- B. Stuart, Infrared Spectroscopy: Fundamentals and Applications, Wiley, New York, 2004 Search PubMed.
- P. Dawson, D. Hirt, J. Rieck, J. Acton and A. Sotthibandhu, Food Res. Int., 2003, 36, 959 CrossRef CAS.
- A. Asadinezhad, I. Novak, M. Lehocky, V. Sedlarik, A. Vesel, I. Junkar, P. Saha and I. Chodak, Colloids Surf., B, 2010, 77, 246 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01233c |
| ‡ Hamidreza Hajfarajollah and Saeid Mehvari contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |