Fast and low-temperature synthesis of one-dimensional (1D) single-crystalline SbSI microrod for high performance photodetector†
Abstract
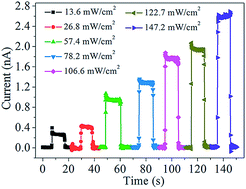
A new rapid and low temperature hydrothermal process has been developed to synthesize one-dimensional (1D) single-crystalline SbSI microrods at 160 °C for 4 h with high quality. Moreover, a first individual SbSI microrod based photodetector equipped with indium–tin-oxide (ITO) electrodes is constructed on a SiO2/Si substrate. The resulting device displays a remarkable response to visible light with an on–off ratio up to 727, a detectivity of 2.3 × 108 Jones, a noise equivalent power of 1.7 × 10−10 W/Hz1/2 and fast response/recovery times (<0.3 s).

 Please wait while we load your content...
Please wait while we load your content...