Structural interpretation, growth mechanism and optical properties of ZnO nanorods synthesized by a simple wet chemical route†
Abstract
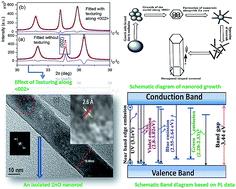
ZnO nanorods are synthesized at room temperature through a simple chemical process without using any template or capping agent. ZnO nanopowders used in this synthesis are synthesized by mechanically alloying the ZnO powder. Here, we report primarily the crystal structure and microstructure interpretations of ZnO nanorods by analyzing X-ray diffraction patterns employing Rietveld refinement, field emission scanning electron microscopy (FESEM) and high resolution transmission electron microscopy (HRTEM) with energy dispersive X-ray (EDX) spectroscopy techniques. Uniformly shaped pure ZnO nanorods with different lengths and diameters are synthesized within 5 h of reaction time. The Rietveld refinement and HRTEM images ascertain the growth of ZnO nanorods along the <002> plane. STEM-HAADF images and EDX spectra and imaging of nanorods confirm the chemical composition and reveal the uniform elemental distributions of Zn and O over the entire nanorod. UV-visible spectra analyses of ZnO nanopowder and nanorods reveal a small decrease in optical band gap of nanorods due to morphological change. Photoluminescence (PL) spectra of both powder and rod-shaped ZnO reveal the presence of excess of oxygen in nanorods. Rietveld analysis corroborates the findings of PL and quantifies the content of oxygen in ZnO nanorods.

 Please wait while we load your content...
Please wait while we load your content...