Development of a monoclonal antibody-based ELISA for the detection of the novel insecticide cyantraniliprole†
Abstract
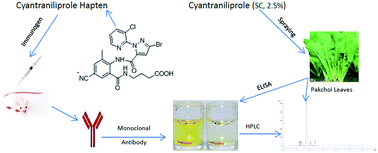
Cyantraniliprole is the newest anthranilic diamide insecticide acting on ryanodine receptors with higher efficacy, and broader spectrum than chlorantraniliprole in pest control. To study the effects of its residues on the environment and food samples, an enzyme-linked immunosorbent assay (ELISA) has been developed. The hapten was synthesized and conjugated with bovine serum albumin (BSA) and ovalbumin (OVA) as the immunogen and coating antigen, respectively. A sensitive and specific monoclonal antibody (mAb) was generated and was designated as mAb3B2. mAb3B2 was used to develop an indirect competitive ELISA (icELISA). The concentration of cyantraniliprole producing 50% inhibition (IC50) was 1.57 μg L−1 and the effective range of icELISA was 0.43–6.15 μg L−1. The icELISA showed low cross-reactivity with chlorantraniliprole, and very low or no cross-reactivity with the other cyantraniliprole analogues. Average recoveries from pakchoi (Brassica rapa) and tap water samples were 93.7–101.0% and 94.4–100.6%, respectively. Meanwhile, to confirm the results of icELISA, high-performance liquid chromatography was applied for the determination of cyantraniliprole residues in pakchoi samples. The comparable results suggest that the ELISA is suitable for rapid detection of cyantraniliprole residues in environmental and agricultural samples.

 Please wait while we load your content...
Please wait while we load your content...