Peroxide detected in imidazolium-based ionic liquids and approaches for reducing its presence in aqueous and non-aqueous environments†
Abstract
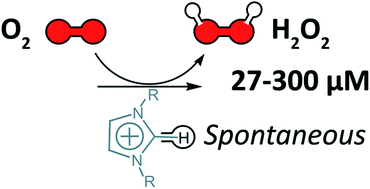
Imidazolium-based ionic liquids were discovered to contain micromolar (μM) quantities of a peroxide species. A general approach using catalase (aqueous solution) or a salen–manganese complex (neat IL) for reducing the presence of the peroxide species is described herein.

 Please wait while we load your content...
Please wait while we load your content...