A facile approach to prepare biomimetic composite separators toward safety-enhanced lithium secondary batteries
Abstract
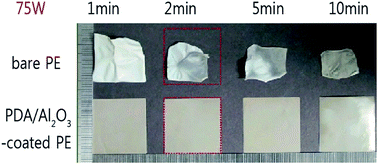
A mussel-inspired polydopamine (PDA) coating turns radio-frequency (RF) Al2O3 sputtering – which thus far has not been appropriate for the surface treatment of porous polyolefin-based separators – into a damage-free, reliable, and cost-efficient process. Due to the thermally resistive PDA layers, polyethylene (PE) separators can sustain high-power Al2O3 sputtering conditions over 75 W, which significantly reduces processing time. Furthermore, compared to the as-prepared separators, PDA/Al2O3-coated PE separators also reveal improved thermal stability and cycle performance for lithium secondary batteries. PDA/Al2O3-coated PE separators retained their original size when exposed to temperatures of 145 °C over 30 min, while the bare PE separators shrank to 9% of their original size. At a temperature of 25 °C, the unit cell (LiMn2O4/separator/Li metal) employing the PDA/Al2O3-coated PE separators maintained 94.8% (103.4 mA h g−1) of the initial discharge capacity after 500 cycles at C/2 rate and 51.7% (56.7 mA h g−1) at 25 C rate, while the corresponding values for the bare PE separators were 89% (98.6 mA h g−1) at C/2 rate and 24.5% (27.2 mA h g−1) at 25 C rate.

 Please wait while we load your content...
Please wait while we load your content...