Two Schiff base ligands for distinguishing ZnII/CdII sensing—effect of substituent on fluorescent sensing†
Abstract
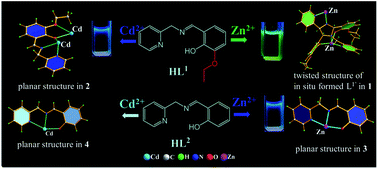
Two Schiff base ligands (HL1, HL2) were conveniently synthesised by one-step condensation between pyridine 2-ylmethanamine and 3-ethoxy-2-hydroxybenzenaldhyde (for HL1) or salicylaldehyde (for HL2) as fluorescent sensors for distinguishing sensing of Zn2+ or Cd2+. Both of the two fluorescent sensors present very weak emission at 463 nm (for HL1) or 453 nm (for HL2). For HL1, upon addition of Zn2+, the fluorescence intensity of HL1 enhanced and gradually red shifted to 493 nm with a green emission while addition of Cd2+ only induced enhancement of fluorescent intensity at 463 nm. For HL2, only addition of Zn2+ induced enhancement of fluorescence intensity, presenting a high Zn2+/Cd2+ selectivity. A Zn2+-induced red shift in fluorescent spectra of HL1 could be attributed to twisted intramolecular charge transfer (TICT) from the interaction between the Zn2+ ion and in situ formed ligand L1′ with the twisted structure in compound 1, which is absent in compound 2. The Zn2+/Cd2+ selectivity of fluorescent response for HL2 correlates with the Cd–HL2 and Zn–HL2 coordination bond distances. Obviously, introduction of ethoxyl groups onto the benzene ring as an electron-donating group facilitates the Zn-induced in situ dimerization of HL1 into new ligand L1′ with a twisted molecular structure, further resulting in a red shift of the fluorescence spectra.

 Please wait while we load your content...
Please wait while we load your content...