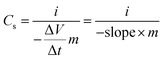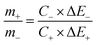Hierarchical NiMn2O4@CNT nanocomposites for high-performance asymmetric supercapacitors†
Honghong Nan,
Wenqin Ma,
Zhengxiang Gu,
Baoyou Geng and
Xiaojun Zhang*
Key Laboratory for Functional Molecular Solids of the Education Ministry of China, College of Chemistry and Materials Science, Center for Nano Science and Technology, Anhui Normal University, Wuhu, 241000, PR China. E-mail: xjzhang@mail.ahnu.edu.cn; Fax: +86-553-3869302; Tel: +86-553-3937135
First published on 23rd February 2015
Abstract
Miniaturized energy storage devices have attracted considerable research attention due to their promising applications in various smart electronic devices. In this work, a high performance asymmetric supercapacitor (ASC) device was designed and fabricated wherein a novel nanocomposite consisting of manganese oxide (NiMn2O4) nanosheets with carbon nanotubes (CNTs) was used as the active material. High capacitance of 151 F g−1 and energy density of 60.69 W h kg−1 were achieved for the CNT@NiMn2O4 nanocomposites ASC at a current density of 1 A g−1, which attributing to the widen operation voltage window ranging from 0 to 1.7 V. Moreover, the CNT@NiMn2O4 nanocomposites ASC also showed remarkable cycling stability with 96.3% energy density retention after 5000 cycles. As a result, the CNT@NiMn2O4 nanocomposite is a possible contender materials for next generation supercapacitors in high energy density storage systems.
1. Introduction
When asked about the most crucial problem facing society today, many people say that it is the serious energy crisis, caused by over usage of fossil-fuel resources and environmental pollution. Considering the increasing consumption of fossil fuels and continuous worsening climate, renewable energy resources have started to take place in our daily lives. In the meanwhile, energy storage systems with high power density and/or high energy density have being a pressing need. On the other hand, with the tremendous development of the portable and wearable electronic devices, there has been an increasing demand for lightweight, environmentally friendly and high performance energy storage/conversion systems.1–5 As the most promising strategies for energy storage, supercapacitors (also known as electrochemical capacitors) have attracted intensive attention because of they not only can instantaneously provide higher power density than batteries and higher energy density than conventional dielectric capacitors which benefit from their outstanding properties: superior power density, high charging and discharging rates, and long cycle life,6–8 but also can be used in foldable and rollup devices.9–12 In recent years, the increasing worldwide interest in carbon materials (CNTs, activated carbon, and graphene) as electrodes for supercapacitors is based on the fact that carbon-based supercapacitors will ultimately serve as a safety and low cost alternative to current commercial electrical double layer supercapacitors (EDLCs). These EDLCs offer high power density and excellent cycling performance but suffer from relatively low capacitance and energy density.13,14 On the other hand, the pseudo-capacitors supercapacitors, with transition metal oxides and/or conductive polymers as active materials, have high energy density with a storage mechanism based on surface electrochemical reaction.15,16 However, due to the grand challenges in device fabrication and limited selections of raw materials, the research of pseudo-capacitors is still a titsinfancy.15,17–23As an important cubic, spinel binary metal oxide, NiMn2O4 is promising electrode materials due to their exceptional chemical stability and outstanding electrochemical capacitance.24 Firstly, the nickel cations almost exclusively occupy the octahedral B-sites in preference to the tetrahedral A-sites of the cubic close packed oxygen sublattice (an inverse spinel).25,26 Secondly, NiMn2O4 can provide high redox active sites owning to the variability of the Ni and Mn lattice positions. These intriguing features make nickel manganese oxide to be investigated widely and extensively in many fields, such as catalysis, negative temperature coefficient thermistors, magnetism, sensors, supercapacitors, etc. However, the utilization of NiMn2O4 in energy storage devices is often hindered by its poor electronic conductivity.27–32 For instance, porous structured NiMn2O4 material has been successfully synthesized by calcining oxalate precursors.33 Spinel nickel manganese oxide with large specific surface area and suitable pore size is also prepared from an peroxide-driven sol–gel process.34 Nano-sized and well dispersed manganese oxide and nickel manganese oxide (Ni–Mn–O) powders are synthesized via the hydrothermal route.35 In order to improve the electrical properties, various approaches have been proposed.36–44 For example, porous electroconductive NiMn2O4/C hierarchical tremella-like nanostructures have been used as anode and showed high lithium storage properties.45 However, despite all these recent progresses have been demonstrated efficiently, they still suffered from complicated method, lower specific capacitance and easily coalesce into group after several cycles, etc. Therefore, it is still an urge to exploit new electrode material with high electroconductive, larger specific surface area and good dispersity.
CNTs are molecular wires that have become the leading building blocks for nanomaterials and have shown great potential in supercapacitors due to their excellent conductivity, very high strength, chemical stability, high specific surface area, and low density. Due to the outstanding characters esp. the electron transfer capability and easy availability at present, the CNTs welled up inside our mind to work as electrode firstly. Although several studies focusing on CNT-supported nanomaterials have been reported, there is still the challenge of the direct growth of NiMn2O4 on CNTs by a facile method.
In this work, we designed and synthesized hierarchical CNT@NiMn2O4 nanocomposites from NiMn2O4 nanosheets and CNTs, where in the CNTs matrix provided electron conductive channels and NiMn2O4 nanosheets provided large surface area for electrochemical reaction. From the perspective of device fabrication and design, we took CNT@NiMn2O4 nanocomposites as a model system to fabricate asymmetric supercapacitors. It is worth mentioning that the CNT@NiMn2O4//Active Carbon (AC) asymmetric supercapacitor shows high energy density of 60.69 W h kg−1 at a power density of 874 W kg−1 as well as long-term stability, outperforming most currently available ASCs.
2. Experimental section
2.1. Chemicals
All the reagents used in the experiment are analytical grade and used as received without further purification. Multiwalled carbon nanotubes (MWNTs-2040, purity of 95%, average diameter of 20–40 nm, and length range 5–15 μm) were obtained from Shenzhen Nanotech Port Co., Ltd. (Shenzhen, China). Acetylene black, active carbon and polytetrafluoroethylene (PTFE) were achieved from Guangzhou Songbai Chemical Industry Co., Ltd. (Guangdong, China). The nickel(II) chloride hexahydrate (NiCl2·6H2O) and potassium permanganate (KMnO4) were purchased from Tianjin Chemical Co., Ltd. (Tianjin, China) All aqueous solutions were freshly prepared with high purity water (18 MΩ resistance).2.2. Multiwalled carbon nanotubes (CNT) immobilized NiMn2O4 nanosheet arrays (CNT@NiMn2O4)
CNT@NiMn2O4 nanocomposites were synthesized by a facile hydrothermal method. The synthetic route is illustrated in Scheme 1. Firstly, the reaction solution was obtained by mixing 1 mg CNT, 0.5 mmol nickel(II) chloride hexahydrate, 1 mmol potassium permanganate in 20 mL of distilled water under magnetic stirring for 1 h in air. Then the resulting solution was transferred into a 60 mL Teflon-lined stainless steel autoclave. After keeping 6 h at 100 °C, the autoclave was then naturally cooled down to room temperature. Subsequently, in order to remove the free debris and residual reactant, the mixture was centrifuged and washed several times with deionized water and absolute alcohol. Finally, the as-synthesized CNT@NiMn2O4 core–shell nanosheet arrays was dried at 80 °C for 6 h in an air oven to obtained the products.2.3. Materials characterization
The crystal structure of CNT@NiMn2O4 nanocomposites was measured with X-ray diffraction (XRD). XRD patterns were taken from 10° to 80° with a continuous scan mode to collect 2θ data using Cu Kα radiation. The chemical composition of the product was examined by means of energy dispersive X-ray spectroscopy (EDX, S-4800, Hitachi Co., Ltd., Tokyo, Japan). The interfacial property of the nanosheet arrays was examined with a scanning electron microscope (SEM, S-4800, Hitachi Co., Ltd., Tokyo, Japan). The morphologies of the carbon nanotubes immobilized NiMn2O4 nanoparticles were characterized with a JEM-1200 EX/S transmission electron microscope (TEM). Powders were dispersed in ethyl alcohol in an ultrasonic bath for 5 min and then deposited on a copper grid covered with a perforated carbon film. X-ray photoelectron spectroscopy (XPS) analysis was performed in a VG ESCALAB 220i-XL UHV surface analysis system with a monochromatic Al Kα X-ray source (1486.6 eV). The BET specific surface area and the pore size distribution of the sample were investigated by the Brunauer–Emmett–Teller (BET) and the Barrett–Joyner–Halenda (BJH) methods.2.4. Preparation of working electrode
The working electrodes were prepared by coating mixtures of the CNT@NiMn2O4 nanocomposites, acetylene black, and polytetrafluoroethylene binder (weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on a slice of nickel foil current collector (1 mm thick). Working electrode with a geometric surface area of about 1 cm2 contained about 2 mg of CNT@NiMn2O4 nanocomposites. The as-prepared electrode was dried at 80 °C for 12 h in vacuum.
1) on a slice of nickel foil current collector (1 mm thick). Working electrode with a geometric surface area of about 1 cm2 contained about 2 mg of CNT@NiMn2O4 nanocomposites. The as-prepared electrode was dried at 80 °C for 12 h in vacuum.
2.5. Electrochemical measurements
The electrochemical measurements of the CNT@NiMn2O4 nanocomposites electrode were carried out in a three-electrode electrochemical cell containing 6 M KOH aqueous solution as the working electrolyte with a platinum wire and Ag/AgCl electrode as the counter and reference electrode, respectively. The cyclic voltammetry (CV) and galvanostatic charge–discharge measurements were performed on a CHI 760D electrochemical workstation (Chenhua, Shanghai, China) in the potential range of −0.2–0.5 V.To further evaluate the CNT@NiMn2O4 nanocomposite for real device applications, we fabricated asymmetric capacitors (ACS) in two-electrode test system with 6 M KOH as the electrolyte. The ASC was assembled using CNT@NiMn2O4 nanocomposites and commercially available activated carbon (AC) as positive and negative electrodes, respectively. Particularly, the negative electrode was prepared as follows: first, 90 wt% activated carbon and 10 wt% polyvinylidene fluoride binder dispersed in 1-methylpyrrolidone solvent were mechanically mixed to produce a homogeneous paste. Then the mixture was coated onto the nickel foil substrate to form the electrode layer. Finally, the fabricated electrode was pressed and then dried at 60 °C for 12 h. The electrochemical performances of the ASC device were measured by the same procedure as mentioned above. All the electrochemical measurements were conducted at room temperature.
3. Results and discussion
3.1. Characterizations
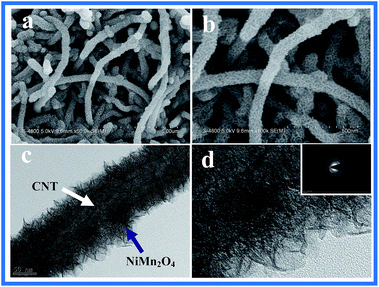
Morphology of the synthesized CNT@NiMn2O4 nanocomposites was investigated by SEM. The low-magnification micrograph (see Fig. 1a) reveals that numerous NiMn2O4 nanosheet were found well-grown around individual CNTs to form a conformal coating on the surface. From the enlarged view (see Fig. 1b), the nanosheet are in mutual contact, which might give better mechanical strength and form a better conductive network not easily detached from the CNT core. Such a nanostructure may bring significant benefit by an increase in its specie area while maintaining a conductive core, which is of great importance to the electrochemical performance of electrode materials.46 Further insight into the detailed nanostructure is elucidated by TEM. In Fig. 1c and d, the hybrid oxide nanoparticles consisting of nanocrystallites were uniformly grown on the surface of the CNTs backbone. The above mentioned results show that CNTs are highly suitable substrates for spinel nickel manganese's nucleation and crystal growth. Furthermore, the unique features mentioned above can make CNT@NiMn2O4 nanocomposites accessible to electrolytes to a larger extent, which makes the nanostructures potential candidates as electrodes for supercapacitors with high capacitance and excellent cycling stability.47 The XRD pattern (see Fig. 2a) exhibits several distinct peaks corresponding to the (111), (222), (400) and (422) planes in the standard NiMn2O4 spectrum (JCPDS 44-0141). The additional peaks at 25.6°, 43.9°, 63.5° and 77.5° can be attributed to the (002), (100), (440) and (006) reflections of CNTs, confirming the successful integration of NiMn2O4 with CNTs. It is worth mentioning that no other peaks are observed, which effectively confirms the purity of the obtained NiMn2O4 phase, which was supported on one-dimensional CNT. The EDS measurement (see Fig. S1†) shows that the molar ratio of Ni to Mn is almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which is in accordance with the result of XRD. Oxidation state of the corresponding transition metal ions in the obtained sample is further investigated with X-ray photoelectron spectroscopy (XPS). A survey spectrum (Fig. 2b) shows the presence of Ni, Mn, and O as well as C and there is no other impurity. A Mn 2p core level spectrum (Fig. 2c) shows two major peaks with binding energies of 641.7 and 653.2 eV, assigned to the Mn 2p3/2 and Mn 2p1/2 peaks, respectively.48 After refined fitting, the spectrum composes of four peaks. Those with binding energies of 641.8 eV and 653.3 eV are ascribed to Mn3+. Another two peaks at 640.7 eV and 652.3 eV are ascribed to Mn2+. Similarly, the Ni 2p spectrum (Fig. 2d) shows two spin–orbit doublets characteristics of Ni2+ and Ni3+ states and shake-up peaks at around 861.2 and 879.7 eV at the high binding energy side of the Ni 2p3/2 and Ni 2p1/2 edge,49,50 The fitted peaks at 854.5 and 872.1 eV are attributed to Ni2+, while the other peaks at 855.8 and 874.0 eV are related to Ni3+. In the C 1s spectrum (Fig. 2e) and O 1s spectrum (Fig. 2f) the peaks are consistent with the reported values.51 According to the XPS analyses, the couples of Mn3+/Mn2+ and Ni3+/Ni2+ are coexisting in the spinel NiMn2O4 nanostructures. The atomic ratio of Ni and Mn is ∼1
2, which is in accordance with the result of XRD. Oxidation state of the corresponding transition metal ions in the obtained sample is further investigated with X-ray photoelectron spectroscopy (XPS). A survey spectrum (Fig. 2b) shows the presence of Ni, Mn, and O as well as C and there is no other impurity. A Mn 2p core level spectrum (Fig. 2c) shows two major peaks with binding energies of 641.7 and 653.2 eV, assigned to the Mn 2p3/2 and Mn 2p1/2 peaks, respectively.48 After refined fitting, the spectrum composes of four peaks. Those with binding energies of 641.8 eV and 653.3 eV are ascribed to Mn3+. Another two peaks at 640.7 eV and 652.3 eV are ascribed to Mn2+. Similarly, the Ni 2p spectrum (Fig. 2d) shows two spin–orbit doublets characteristics of Ni2+ and Ni3+ states and shake-up peaks at around 861.2 and 879.7 eV at the high binding energy side of the Ni 2p3/2 and Ni 2p1/2 edge,49,50 The fitted peaks at 854.5 and 872.1 eV are attributed to Ni2+, while the other peaks at 855.8 and 874.0 eV are related to Ni3+. In the C 1s spectrum (Fig. 2e) and O 1s spectrum (Fig. 2f) the peaks are consistent with the reported values.51 According to the XPS analyses, the couples of Mn3+/Mn2+ and Ni3+/Ni2+ are coexisting in the spinel NiMn2O4 nanostructures. The atomic ratio of Ni and Mn is ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 based on the areas of their corresponding XPS peaks.
2 based on the areas of their corresponding XPS peaks.
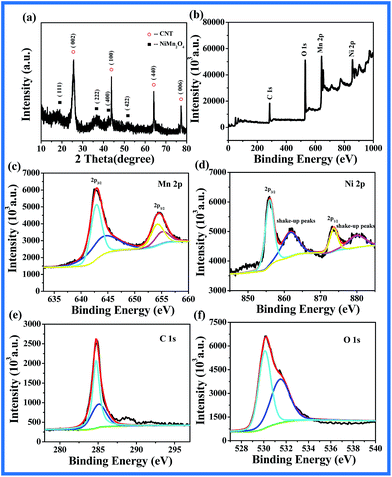 | ||
| Fig. 2 (a) XRD patterns of CNT@NiMn2O4 nanocomposites; XPS spectra for the as-prepared CNT@NiMn2O4 nanocomposites: (b) a survey spectrum, (c) Mn 2p, (d) Ni 2p, (e) C 1s and (f) O 1s. | ||
In addition, the porous characteristics of the above mentioned sample were further studied by nitrogen adsorption and desorption measurement, as presented in Fig. 3. Fig. 3a shows the adsorption–desorption isotherm of CNT@NiMn2O4 nano-composites. It is a typical type III isotherm, which could be attributed to N2 adsorption–desorption in metallic oxide structures. The calculated specific surface area of the CNT@NiMn2O4 nanocomposites is 237.8 m2 g−1, according to the BET test. The large specific area could greatly increase the utilization of NiMn2O4 as an electrochemically active material in electrodes.52 Fig. 3b shows the corresponding pore size distribution calculated by the BJH model. As can be seen, the product possesses a wide pore size distribution, which is attributed to the mesoporous structure of NiMn2O4 nanosheets and the large pores between these nanosheets. This hierarchically porous structure facilitates the mass transportation of electrolytes within the bulk of electrode materials, promoting electrolyte accessibility, kinetic reversibility, and electrochemical reaction homogeneity.53–55
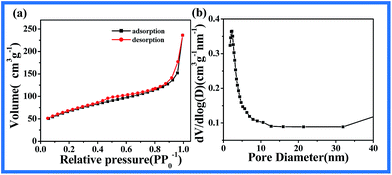 | ||
| Fig. 3 (a) N2 adsorption–desorption isotherm of CNT@NiMn2O4 nanocomposites; (b) BJH measurement of CNT@NiMn2O4 nanostructure. | ||
3.2. Electrochemical studies
where i is the current applied, ΔV/Δt is the slope of the discharge curve after the iR drop, and m is the mass of CNT@NiMn2O4 nanocomposites.
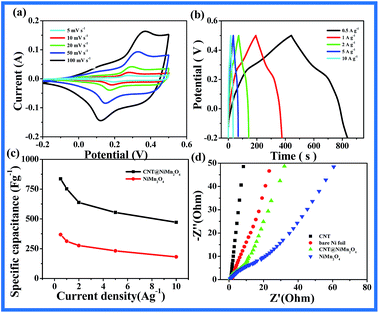
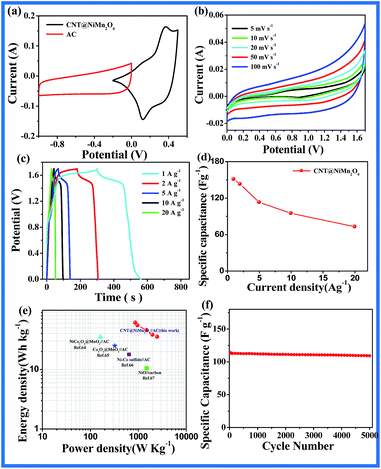
The calculated rate performance of the material is shown in Fig. 4c, it can be observed that the specific capacitance can attain a maximum of 836 F g−1 at a current density of 0.5 A g−1. Even at current density as high as 10 A g−1, 471 F g−1 can still be retained, which suggests that about 44.6% of the specific capacitance is lost when the current density increases from 0.5 A g−1 to 10 A g−1. For comparison, the pure NiMn2O4 nanosheet shows lower capacitance and poorer rate performance at the same conditions (as shown in Fig. 4c), the capacitance of the powder decreases from 368 F g−1 (at 0.5 A g−1) to 181 F g−1 (at 10 A g−1), with a reduction of 50.9%. It is easy to conclude that the rate capacitance of CNT@NiMn2O4 is superior to that of pure NiMn2O4. The superior electrochemical performance of CNT@NiMn2O4 nanocomposites should be attributed to the large surface area of NiMn2O4 nanosheet shell and highly conductive CNT core.
EIS measurements were also carried out to evaluate the charge transfer and electrolyte diffusion in the electrode/electrolyte interface, as shown in Fig. 4d. Obviously, the EIS of the CNT@NiMn2O4 nanocomposites and bare NiMn2O4 electrode was composed of a semicircle at the high-frequency region and a straight line at the low-frequency region. Obviously, the CNT@NiMn2O4 nanocomposites electrode exhibits slightly lower diffusive resistance (Warburg impedance) than bare NiMn2O4 electrode at the low-frequency region. However, compared to CNT and Ni foil electrode materials, the CNT@NiMn2O4 nanocomposites electrode had relatively larger diffusive resistance. Since Warburg resistance is related to the ion diffusion/transport in the electrolyte, the lower Warburg resistance will facilitate the diffusion of electrolyte ions (OH−) into the electrodematerials,59 accordingly, a facile and reversible Faradaic redox would be taken,60 and a good electrochemical property could be realized for the CNT@NiMn2O4 nanocomposites electrode.
Subsequently, to ensure the charge balance of the hybrid cell, the optimal weight ratio between the positive and negative electrodes is 0.89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the active mass of the ASC includes materials of both the anode and cathode electrodes. To estimate the stable potential window of CNT@NiMn2O4//AC ASC, CV measurements were performed on the two electrode materials in 6 M KOH aqueous solution before evaluating the asymmetric cell, using a three-electrode system with a platinum wire as counter electrode and Ag/AgCl electrode as reference electrode.
1, and the active mass of the ASC includes materials of both the anode and cathode electrodes. To estimate the stable potential window of CNT@NiMn2O4//AC ASC, CV measurements were performed on the two electrode materials in 6 M KOH aqueous solution before evaluating the asymmetric cell, using a three-electrode system with a platinum wire as counter electrode and Ag/AgCl electrode as reference electrode.
As shown in Fig. 5a, the CNT@NiMn2O4 nanocomposites electrode is measured within a potential window of −0.2 to 0.5 V, while the activated carbon (AC) electrode is measured within a potential window of −1 to 0 V at a scan rate of 100 mV s−1. The CV curve of the activated carbon electrode exhibits a nearly rectangular shape, which is a characteristic of an electric double layer capacitor. As for the CNT@NiMn2O4 nanocomposites, two redox peaks are easily observed, which have already been analyzed above. On the basis of these results, it can be seen that the potential window of CNT@NiMn2O4 nanocomposites electrode is from −0.2 to 0.5 V and the active carbon is from −1 to 0 V with capacitive behavior. Furthermore, it can be observed that the two materials are stable in a different range of potentials. Therefore, the overall capacitance of the CNT@NiMn2O4//AC ASC device derives from the combined contribution of electrical double layer capacitance and redox pseudocapacitance. The total operating cell voltage can be expressed as the sum of the potential range for the two electrodes. It is possible to conclude that the cell potential can be extended up to 1.7 V in 6 M KOH aqueous solution, if the two electrodes are assembled into ASC devices. Therefore, we construct an ASC device by using CNT@NiMn2O4 as the positive electrode and activated carbon (AC) as the negative electrode in a 6 M KOH electrolyte. Fig. 5b shows the CV curves of our ASC device in a potential window of 0–1.7 V in 6 M KOH electrolyte at different scan rates of 5, 10, 20, 50, and 100 mV s−1. Compared to that of pure CNT@NiMn2O4 and activated carbon electrode, the ASC device shows relatively rectangular CV curves without obvious redox peaks, even at the potential up to 1.7 V, indicating the ideal capacitive behavior. In addition, CV profiles still retain a relatively rectangular shape without obvious distortion with increasing potential scan rates, indicating the desirable fast charge–discharge property for power devices. Rate capability is an important factor for the electrochemical capacitors in power applications. So we perform galvanostatic charge–discharge tests at various current densities in 6 M KOH electrolyte. A good electrochemical energy storage device is required to provide its high specific capacitance and high energy density at a high charge–discharge rate. Plots of voltage versus time for the ASC at various current densities are shown in Fig. 5c. Fig. 5d presents the relationship between the specific capacitance of the ASC device and current density. As we can seen, the ASC device delivers a maximum specific capacitance of 151 F g−1, 147 F g−1, 113.5 F g−1, 94.7 F g−1 and 72.8 F g−1 at the current density of 1 A g−1, 2 A g−1, 5 A g−1, 10 A g−1 and 20 A g−1, respectively. The specific capacitance drops 66.9% when the current density increases from 1 to 20 A g−1. The specific capacitance gradually decreases with the increase of current density, since diffusion most likely limits the movement of ions and electrons due to the time constraint. Fig. 5e shows the Ragone plot of the ASC device measured in the voltage window of 0–1.7 V. The energy and power densities are derived from the discharge curves at different current densities. For comparison, the asymmetric supercapacitor devices on CNT@NiMn2O4//AC, NiCo2O4@MnO2//AC,64 Co3O4@MnO2//AC,65 Ni–Co sulfide//AC66 and NiO//carbon67 are assembled and characterized. Specific capacitance of these ASC devices have lower energy and power densities than the CNT@NiMn2O4//AC ASC devices. The cycling stability of the asymmetric supercapacitor was further investigated by galvanostatic charge–discharge between 0 and 1.7 V at a current density of 5 A g−1 (Fig. 5f). After galvanostatic charge–discharge for 5000 cycles, the ASC device presents a capacitance of 109.3 F g−1 with 96.3% of the maximum value, indicating that the high stability of our ASC device is suitable for high-performance supercapacitor applications.
Lightweight and flexible supercapacitors that have excellent electrochemical performance even under mechanical deformation are highly desired to meet the demands of future development of multifunctional flexible electronics. The schematic diagram of the asymmetric ASC is illustrated in Fig. 6a. In order to explore the potential application of the CNT@NiMn2O4//AC ASC as a power source, the ASC could power many colors LEDs after charging for only 30 s (Fig. 6b and c).
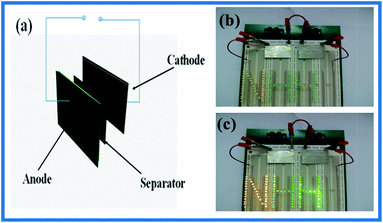 | ||
| Fig. 6 (a) Schematic diagram of a solid-state CNT@NiMn2O4//AC ACS; LED powered before (b) and after (c) charged tandem ASC devices. | ||
Several contributing factors are considered for the high specific capacitance and high energy density of the ASC device: firstly, the energy density of electrochemical capacitors is proportional to the square of operating voltage. The voltage range of our ASC devise is extended to 1.7 V, consequently resulting in a significantly increase in energy density. Secondly, the CNT@NiMn2O4 nanocomposites with large specific area ensures the high utilization of active materials, fast ion and electron transfer, and good structural stability, further decreasing the kinetic limitations of the pseudocapacitive electrode and hence resulting in the high specific capacitance and energy density of our ASC device. Thirdly, the carbon material in the cathode electrode, which is widely used as a conductive additive in electrochemical systems, possesses excellent electric conductivity as an electric double layer capacitor electrode. Apart from, the synergistic effect of the two electrodes is also an important contributing factor of the excellent electrochemical performances of the ASC device.
4. Conclusions
A highly crystalline CNT@NiMn2O4 nanocomposites was synthesized by a facile hydrothermal method. The CNT@NiMn2O4 nanocomposites electrode delivers noticeable pseudocapacitive performance with a high capacitance of 836 F g−1 at 0.5 A g−1. For more practical application, a novel and durable ASC on CNT@NiMn2O4//AC is fabricated, the device shows electrochemical capacitance performance within a voltage range of 0–1.7 V and exhibits high specific capacitance from 151 to 72.8 F g−1 as the current density increases from 1 to 20 A g−1, even specific capacitance retention of 96.3% after 5000 cycles at 5 A g−1, as well as high energy density and good electrochemical stability, the ASC could power many colors LEDs after charging for only 30 s. The CNT@NiMn2O4 nanocomposites is expected to be a promising candidate for application in high-performance electrochemical capacitors, and the as-fabricated ASC also shows great potential in the development of energy storage devices with high energy and power densities.Acknowledgements
This work was financially supported by the projects (no. 21371007) from National Natural Science Foundation of China, Anhui Provincial Natural Science Foundation (1208085QB28), Anhui Provincial Natural Science Foundation for Distinguished Youth (1408085J03), Natural Science Foundation of Anhui (KJ2012A139) and the Program for Innovative Research Team at Anhui Normal University.Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- R. Kotz and M. Carlen, Electrochim. Acta, 2000, 45, 2483 CrossRef CAS.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- C. H. Xu, B. H. Xu, Y. Gu, Z. G. Xiong, J. Sun and X. S. Zhao, Energy Environ. Sci., 2013, 6, 1388 CAS.
- Z. Y. Guo, J. Wang, F. Wang, D. D. Zhou, Y. Y. Xia and Y. G. Wang, Adv. Funct. Mater., 2013, 23, 4745 Search PubMed.
- S. Boukhalfa, K. Evanoff and G. Yushin, Energy Environ. Sci., 2012, 5, 6872 CAS.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, X. L. Wang, C. D. Gu, X. B. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- H. Wu, M. Xu, H. Y. Wu, J. J. Xu, Y. L. Wang, Z. Peng and G. F. Zheng, J. Mater. Chem., 2012, 22, 19821 RSC.
- C. W. Cheng and H. J. Fan, Nano Today, 2012, 7, 327 CrossRef CAS PubMed.
- L. Nyholm, G. Nyström, A. Mihranyan and M. Strømme, Adv. Mater., 2011, 23, 3751 CAS.
- G. Nyström, A. Razaq, M. Strømme, L. Nyholm and A. Mihranyan, Nano Lett., 2009, 9, 3635 CrossRef PubMed.
- D. P. Dubal, S. H. Lee, J. G. Kim, W. B. Kim and C. D. Lokhande, J. Mater. Chem., 2012, 22, 3044 RSC.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774 RSC.
- D. N. Futaba, K. Hata, T. Yamada, T. Hiraoka, Y. Hayamizu, Y. Kakudate, O. Tanaike, H. Hatori, M. Yumura and S. Iijima, Nat. Mater., 2006, 5, 987 CrossRef CAS PubMed.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11 CrossRef CAS PubMed.
- X. Lang, A. Hirata, T. Fujita and M. Chen, Nat. Nanotechnol., 2011, 6, 232 CrossRef CAS PubMed.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760 CrossRef CAS PubMed.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073 CrossRef CAS.
- E. Frackowiak and F. Beguin, Carbon, 2002, 40, 1775 CrossRef CAS.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238 CAS.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697 RSC.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1 CrossRef CAS PubMed.
- R. B. Rakhi, W. Chen, D. Cha and H. N. Alshareef, Nano Lett., 2012, 12, 2559 CrossRef CAS PubMed.
- M. Toupin, T. Brousse and D. Belanger, Chem. Mater., 2004, 16, 3184 CrossRef CAS.
- W. Chen, R. B. Rakhi, Q. Wang, M. N. Hedhili and H. N. Alshareef, Adv. Funct. Mater., 2014, 24, 3130 CrossRef CAS.
- C. Yuan, L. Hou, L. Yang, D. Li, L. Shen, F. Zhang and X. Zhang, J. Mater. Chem., 2011, 21, 16035 RSC.
- J. M. Schnorr and T. M. Swager, Nanostructured Thermoelectrics: The New Paradigm, Chem. Mater., 2010, 23, 646 CrossRef.
- I. Dumitrescu, P. R. Unwin and J. V. Macpherson, Chem. Commun., 2009, 6886 RSC.
- X. Chen, H. Zhu, Y. C. Chen, Y. Shang, A. Cao, L. Hu and G. W. Rubloff, ACS Nano, 2012, 6, 7948 CrossRef CAS PubMed.
- X. Tang, H. Li, Z.-H. Liu, Z. Yang and Z. Wang, J. Power Sources, 2011, 196, 855 CrossRef CAS PubMed.
- G. Qiu, H. Huang, S. Dharmarathna, E. Benbow, L. Stafford and S. L. Suib, Chem. Mater., 2011, 23, 3892 CrossRef CAS.
- W. Zhang, J. Pu, B. Chi and L. Jian, J. Power Sources, 2011, 196, 5591 CrossRef CAS PubMed.
- D. L. Fang, Z. B. Wang, P. H. Yang, W. Liu, C. S. Chen and A. J. Winnubst, J. Am. Ceram. Soc., 2006, 89, 230 CrossRef CAS PubMed.
- J. A. Schmidt, A. E. Sagua, J. C. Bazán, M. R. Prat, M. E. Braganza and E. Morán, Mater. Res. Bull., 2005, 40, 635 CrossRef CAS PubMed.
- X. Wang, X. Han, M. Lim, N. Singh, C. L. Gan, M. Jan and P. S. Lee, J. Phys. Chem. C, 2012, 116, 12448 CAS.
- H. Wang, C. M. B. Holt, Z. Li, X. Tan, B. S. Amirkhiz, Z. Xu, B. C. Olsen, T. Stephenson and D. Mitlin, Nano Res., 2012, 5, 605 CrossRef CAS.
- J. Du, G. Zhou, H. Zhang, C. Cheng, J. Ma, W. Wei, L. Chen and T. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 7042 Search PubMed.
- X. Wang, W. S. Liu, X. Lu and P. S. Lee, J. Mater. Chem., 2012, 22, 23114 RSC.
- X. Zhang, J. Zhang, R. Wang and Z. Liu, Carbon, 2004, 42, 1455 CrossRef CAS PubMed.
- T. Zhu, B. Xia, L. Zhou and X. W. Lou, J. Mater. Chem., 2012, 22, 7851 RSC.
- T. Zhu, H. B. Wu, Y. Wang, R. Xu and X. W. Lou, Adv. Energy Mater., 2012, 2, 1497 CrossRef CAS.
- J. F. Li, S. L. Xiong, X. W. Li and Y. T. Qian, Nanoscale, 2013, 5, 2045 RSC.
- J. F. Marco, J. R. Gancedo, M. Gracia, J. L. Gautier, E. I. Ríos, H. M. Palmer, C. Greaves and F. J. Berry, J. Mater. Chem., 2001, 11, 3087 RSC.
- B. Cui, H. Lin, Y. Z. Liu, J. B. Li, P. Sun, X. C. Zhao and C. J. Liu, J. Phys. Chem. C, 2009, 113, 14083 CAS.
- J. Pu, J. Wang, X. Q. Jin, F. L. Cui, E. H. Sheng and Z. H. Wang, Electrochim. Acta, 2013, 106, 226 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., 2008, 47, 2930 CrossRef CAS PubMed.
- R.-T. Wang, L.-B. Kong, J.-W. Lang, X.-W. Wang, S.-Q. Fan, Y.-C. Luo and L. Kang, J. Power Sources, 2012, 217, 358 CrossRef CAS PubMed.
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976 CrossRef CAS PubMed.
- Y. Hou, Y. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727 CrossRef CAS PubMed.
- H. Wang, Q. Gao and L. Jiang, Small, 2011, 7, 2454 CAS.
- C. Yuan, X. Zhang, L. Su, B. Gao and L. Shen, J. Mater. Chem., 2009, 19, 5772 RSC.
- W.-M. Zhang, X.-L. Wu, J.-S. Hu, Y.-G. Guo and L.-J. Wan, Adv. Funct. Mater., 2008, 18, 3941 CrossRef CAS.
- M. Liu, L. Kong, C. Lu, X. Li, Y. Luo and L. Kang, ACS Appl. Mater. Interfaces, 2012, 4, 4631 CAS.
- S. K. Meher and G. R. Rao, J. Phys. Chem. C, 2011, 115, 15646 CAS.
- P. Chen, G. Z. Shen, Y. Shi, H. T. Chen and C. W. Zhou, ACS Nano, 2010, 4, 4403 CrossRef CAS PubMed.
- V. Khomenko, E. Raymundo-Piñero and F. Beguin, J. Power Sources, 2006, 153, 183 CrossRef CAS PubMed.
- X. Du, C. Y. Wang, M. M. Chen, Y. Jiao and J. Wang, J. Phys. Chem. C, 2009, 113, 2643 CAS.
- K. B. Xu, W. Y. Li, Q. Liu, B. Li, X. J. Liu, L. An, Z. G. Chen, R. J. Zou and J. Q. Hu, J. Mater. Chem. A, 2014, 2, 4795 CAS.
- M. Huang, Y. X. Zhang, F. Li, L. L. Zhang, Z. Y. Wen and Q. Liu, J. Power Sources, 2014, 259, 98 CrossRef CAS PubMed.
- Y. H. Li, L. J. Cao, L. Qiao, M. Zhou, Y. Yang, P. Xiao and Y. H. Zhang, J. Mater. Chem. A, 2014, 2, 6540 CAS.
- D. W. Wang, F. Li and H. M. Cheng, J. Power Sources, 2008, 185, 1563 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00979k |
| This journal is © The Royal Society of Chemistry 2015 |