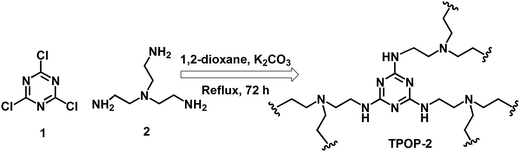
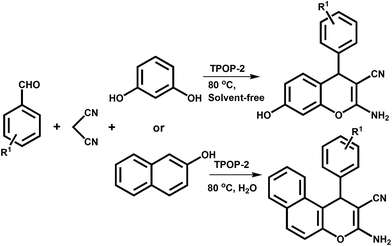
A triazine-based porous organic polymer: a novel heterogeneous basic organocatalyst for facile one-pot synthesis of 2-amino-4H-chromenes†
Sudipta K. Kundu and
Asim Bhaumik*
Department of Materials Science, Indian Association for the Cultivation of Science, Jadavpur 700 032, India. E-mail: msab@iacs.res.in
First published on 27th March 2015
Abstract
A new triazine-based porous organic polymer TPOP-2 has been synthesized through the reaction between cyanuric chloride and tris(2-aminoethyl)amine in anhydrous 1,4-dioxane under N2 atmosphere. The porous polymer has been characterized by powder X-ray diffraction, N2 sorption, HR TEM, FE SEM, 13C CPMAS NMR, CO2-TPD, TG-DTA and FT IR spectroscopic tools. Due to the high density of amine and triazine functional groups, this porous polymer is N-rich and possesses excellent surface basicity, and is utilized as a heterogeneous metal-free basic organocatalyst for the one-pot three-component condensation reaction of aromatic aldehyde, activated phenols (resorcinol and 2-naphthol) and malononitrile for the synthesis of 2-amino-chromenes under solvent-free or aqueous conditions. The ease of catalyst synthesis and its efficient use for five consecutive cycles without noticeable loss in catalytic activity suggest significant future potential of this new N-rich porous organic polymer material for a wide range of base catalyzed reactions.
Introduction
Porous organic polymers (POPs)1 have attracted huge research interest in recent times due to their wide potential to accommodate several reactive functional groups in the framework and these materials are explored in many frontline areas of energy and environmental research, including gas storage and selective separation from a mixture of gases, heterogeneous catalysis, fabricating conductive coatings, sensors, optoelectronic devices, etc. Unique intrinsic surface properties like high BET surface area, good chemical and thermal stability, and reusability in various catalytic reactions have prompted the researchers to explore organic polycondensation reaction routes for designing these porous polymers.2 A wide range of synthetic strategies using bottom-up chemistry approach have been employed to design the functional POPs, that are widely used as heterogeneous catalyst. These led to the discovery of novel porous organocatalytic materials such as microporous organic polymers (MOPs),3 mesoporous poly-melamine-formaldehyde (mPMF),4 polymeric graphitic carbon nitride materials (g-C3N4),5 conjugated microporous polymers (CMPs),6 porous aromatic frameworks (PAFs)7 and related microporous organic polymers.8 Thus, it is a big challenge for the material chemists to explore new synthetic strategies to design a POP material, which can catalyze the complex chemical reaction in a greener, safer and environment friendly way.One-pot multicomponent reactions (MCRs) are very exciting class of reactions in the field of medicinal and synthetic organic chemistry.9 The simplicity of one-pot reaction procedure, conducting the reaction without isolation of intermediates, reduction of reaction time and formation of new C–C, C–O bonds are the main advantages of MCRs compared with traditional reactions. Various heterocyclic compounds such as benzopyrans, benzoxanthene, benzochromene can be synthesized by applying one-pot multicomponent reactions.10 It has been a matter of great interest in recent years to synthesize these chromene derivatives due to their various significant biological and pharmacological activities including antimicrobial,11 antioxidant,12 antitumor,13 central nervous system activity14 and in vitro study as antibacterial agents.15 These compounds have been also employed in the field of cosmetics, pigments and potential biodegradable agrochemicals.16 Owing to these widespread potential biological and pharmacological activities, chromene derivatives are of great interest to the medicinal chemists for many years. 2-Amino-4H-chromenes were generally prepared by the condensation of aromatic aldehyde, malononitrile and activated phenols in presence of hazardous organic bases such as DBU,17 piperidine18 in ethanol and acetonitrile for several hours. There are several reports for the preparation of 2-amino-4H-cromenes over various homogeneous catalysts such as NaOH,19 K2CO3,20 TiCl4,21 InCl3,22 Et3N,23 tetramethylguanidine,24 methane sulfonic acid,25 cetyltrimethylammonium chloride (CTACl),26 triethylbenzylammonium chloride27 and N,N-dimethylaminoethylbenzyl dimethylammonium chloride.28 Although, most of these catalytic methods showed high activity and selectivity but being homogeneous catalysts their separation from the reaction mixture is major problem associated with their no-reusability after one reaction. Only a few heterogeneous catalysts, such as TAFMC-1,29 nano-sized magnesium oxide,30 Mg/Al hydrotalcite,31 γ-alumina32 have been developed for the preparation of 2-amino-4H-chromenes. However, all of the heterogeneous catalysts are metal based and thus having the possibility of metal leaching in the reaction medium. So, greener reaction pathways, methodologies and easily separable heterogeneous organocatalysts, devoid of metal ions are highly desirable to address these industrial and environmental issues.33 Recently, our group has reported mesoporous organosilica as an organocatalyst for the synthesis of 2-amino-4H-chromenes under solvent-free conditions.34 But that organocatalyst gives very low yield of desire product in the cases of resorcinol and 2-naphthol. Thus, high yield synthesis of 2-amino-4H-chromenes under very mild and environment friendly conditions over a tailor-made heterogeneous organocatalyst is highly desirable.
Herein, we report the one-pot three component synthesis of 2-amino-chromene derivatives over a new robust, non-air sensitive heterogeneous metal-free triazine based basic organocatalyst TPOP-2 under solvent-free or aqueous reaction medium. TPOP-2 has been synthesized by the unimolar reaction between cyanuric chloride and tris(2-aminoethyl)amine in anhydrous 1,4-dioxane under N2 atmosphere. The organocatalyst TPOP-2 has been thoroughly characterized by N2 sorption, HR TEM, FE SEM, CO2-TPD, FTIR and 13C CPMAS NMR studies. This novel organocatalyst shows excellent catalytic activity in the one-pot three component condensation reaction of aromatic aldehyde, malononitrile and activated phenols for the synthesis of various 2-amino-chromene derivatives in very high yields.
Experimental section
Synthesis of TPOP-2
In a typical synthesis, 1 mmol tris(2-aminoethyl)amine (2) and 3 mmol anhydrous K2CO3 was added in 10 mL anhydrous 1,4-dioxane taken in 50 mL round bottom flask and it was equipped with dropping funnel containing 1 mmol cyanuric chloride (1) dissolve in 10 mL anhydrous 1,4-dioxane under N2 environment (Scheme 1). Cyanuric chloride, dissolved in 1,4-dioxane was added dropwise to the above solution with continuous stirring at 15 °C for 1 h and it was stirred at 25 °C for 2 h and then the mixture was kept under stirring conditions for 72 h at 90 °C. Finally the yellowish white precipitate was filtered and it was washed several times with water, methanol and acetone. Then the organocatalyst was dried under vacuum condition at room temperature. Yield: 82%. The outline for the synthesis of TPOP-2 is depicted in Scheme 1.General procedure for the synthesis of 2-amino-chromenes
A mixture of 1 mmol of aromatic aldehyde, 1 mmol of activated phenols (resorcinol or 2-naphthol) and 1 mmol of malononitrile was taken in 25 mL round bottom flask with 40 mg TPOP-2 catalyst and then the mixture was heated under solvent-free conditions (when resorcinol was taken) or aqueous medium (when 2-naphthol was taken) at 80 °C for appropriate reaction time (4–6 h). After completion of reaction, ethyl acetate was poured into reaction mixture and the catalyst was separated from the reaction mixture by simple filtration. The products were collected from the reaction mixtures by extracting it with ethyl acetate and water. The organic part was dried with anhydrous sodium sulfate and then under vacuum with reduced pressure to obtain the desired crude product. Then it was crystallised from methanol and pure product was characterised by 1H and 13C NMR spectroscopy. The outline for the base catalyzed condensation reaction is illustrated in Scheme 2.Characterization techniques
Powder X-ray diffraction pattern of TPOP-2 material was recorded on a Bruker D-8 Advance SWAX diffractometer operated at a voltage of 40 kV and a current of 40 mA. The instrument has been calibrated with a standard silicon sample, using Ni-filtered Cu-Kα (λ = 0.15406 nm). FT-IR spectra of the samples were recorded using a Perkin Elmer Spectrum 100. BET surface area and porosity analysis was carried out by using a Quantachrome autosorb-1C at 77 K. JEOL JEM 6700F field emission scanning electron microscope (FE SEM) was used for the determination of morphology of polymer sample. The pore structure was visualized by using a JEOL JEM 2010 high resolution transmission electron microscope (HR TEM) operated at an accelerating voltage of 200 kV. 1H and 13C NMR (solution) experiments were carried out on a Bruker DPX-300 NMR spectrometer. The solid state CP MAS NMR spectra of the samples were taken in Bruker Ascend™ 400 spectrometer. Temperature programmed desorption of carbon dioxide (TPD-CO2) was carried out in a ChemiSorb 2720 pulse chemisorption system of Micromeritics Instrument Corporation, USA. Before purging of CO2 molecules the sample was activated under He flow for 2 h at 150 °C followed by cooling to room temperature.Result and discussion
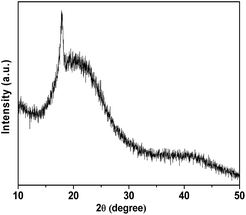
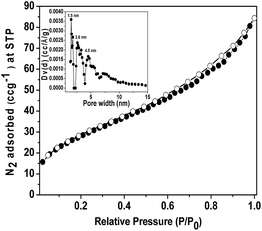
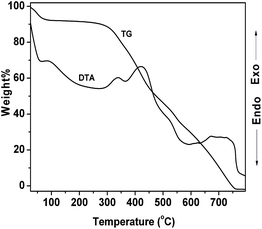
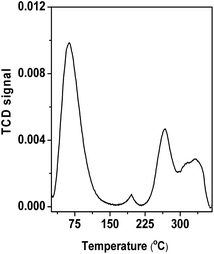
The wide angle powder XRD pattern of TPOP-2 polymer is shown in Fig. 1. It showed one broad peak around at 2θ value of 20 degrees, which is characteristics diffraction by amorphous polymer, along with a sharp peak centred at 2θ value of 18, which suggests partial crystallinity in the porous polymeric network. The detailed porosity of TPOP-2 is measured using N2 adsorption–desorption analysis at 77 K and this is shown in Fig. 2. The isotherm closely resembles the type IV isotherm with a gradual increase in N2 uptake in the intermediate region of P/Po and a noticeable H3 hysteresis loop35 in the range 0.5 to 0.9 of P/Po. From N2 sorption analysis, we get the BET (Brunauer–Emmett–Teller) surface area of TPOP-2 of 105 m2 g−1. Pore size distribution (PSD) of the material has been calculated from non local density functional theory (NLDFT) method. The PSD plot of TPOP-2 (inset, Fig. 2) using carbon slit pore model at 77 K shows trimodal porosity with peaks at 1.5 nm, 2.6 nm and 4.5 nm. The micropore surface area and the micro-pore volume of TPOP-2 are 39 m2g−1 and 0.0187 cc g−1, whereas mesopore surface area is 66 m2 g−1, calculated from the t-method micropore analysis method. We have analyzed the particle morphology of TPOP-2 material via TEM and SEM analysis. Corresponding results are shown in Fig. 3. TEM images (Fig. 3A and B) of TPOP-2 suggested that the typical porous network is formed due to the inter-linking of irregular particles. It clearly reveals the existence small micropores along with mesopores. The scanning electron microscopic images (Fig. 3C and D) of TPOP-2 clearly suggested that the material is composed of spherical nanoparticles of dimension ca. 30–40 nm and they are agglomerated to form bigger particles.The FT IR spectrum of TPOP-2 is shown in Fig. 4. This spectrum shows stretching frequencies of around 1342 and 1572 cm−1 due to the triazine rings.36 On the other hand absorption bands at 1423 and 2938 cm−1 could be attributed to bending and stretching vibration of sp3 –CH2– moiety. The characteristic breathing mode of vibration of triazine units is evident at around 807 cm−1. The absence of C–Cl stretching vibration37 at 850 cm−1 confirms that all three chlorine atoms of cyanuric chloride have been substituted. The elemental analysis of the organocatalyst gave 41.83 wt% C, 6.58 wt% H and 36.27 wt% N. Here nitrogen is the active basic site and thus TPOP-2 can be explored for the base catalyzed condensation reaction. The thermal stability of the porous organocatalyst TPOP-2 is determined from the TG-DTA analysis under continuous flow of N2 flow. The TGA profile is shown in Fig. 5 and it suggests ca. 6% weight loss at 60 °C temperature. This could be due to removal of the adsorbed water from the surface of the porous polymeric network. Then the material is thermally stable up to 340 °C. Two types of exothermic weight loss are observed in the temperature range 340 to 478 °C, one is 6% weight loss up to 350 °C and another is 22% weight loss up to 422 °C. Both weight losses could be attributed to the decomposition of organic fragments of the polymeric network. Further, endothermic weight loss of ca. 26 wt% in the temperature range 486–573 °C is observed. The framework model structure of the TPOP-2 and its 13C CP-MAS NMR are shown in Fig. 6. The 13C CP-MAS NMR spectrum displays characteristic signals for carbon (designated by a) of triazine units at 166.14 ppm. Further, (b) and (c) carbons of the TPOP-2 organocatalyst showed the signals at 39.7 and 52.3 ppm, respectively.38 Temperature programmed desorption of CO2 has been carried out to understand the nature and the amount of surface basicity of the N-rich TPOP-2 surface. Multiple desorption peaks in the CO2-TPD profile (Fig. 7) suggested weak to moderately strong surface basicity of TPOP-2. Total basicity estimated from this CO2 desorption plot is 1.13 mmol g−1.
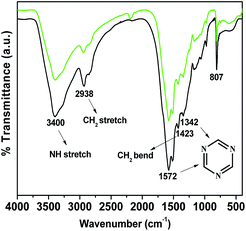 | ||
| Fig. 4 FTIR spectrum of TPOP-2 (black line) and the reused organocatalyst after fourth reaction cycle (green line). | ||
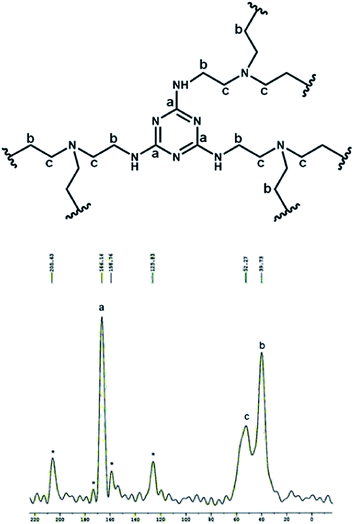 | ||
| Fig. 6 Network bonding of the porous polymer TPOP-2 and its 13C CP-MAS NMR spectrum. The signals marked by ‘*’ symbol are assigned due to the spinning side bands. | ||
Catalysis
The nitrogen rich TPOP-2 can provide abundant basic sites at the catalyst surface and thus we have employed it for the three-component base catalyzed condensation reaction. The catalytic activity of the TPOP-2 is determined by carrying out the reaction of aromatic aldehyde, activated phenols (resorcinol or 2-naphthol) and malononitrile to synthesize 2-amino-chromenes as the model reaction (Table 1). The reaction of aromatic aldehyde, resorcinol and malononitrile is carried out under solvent-free conditions at 80 °C in the presence of the TPOP-2. Very high yield of the products together with considerably good turn over numbers are observed for all the substrates. But when we had taken 2-naphthol instead of resorcinol as the activated phenol under solvent-free conditions very poor yield of 2-amino-chromenes was formed even at 100 °C reaction temperature. To find out the optimized conditions, the unimolar reaction between 4-chlorobenzaldehyde, 2-naphthol and malononitrile is carried out under various reaction mediums over the organocatalyst TPOP-2. Among the various solvents used, such as water, ethanol, acetonitrile, DMF, 1,4-dioxane, n-hexane; this reaction underwent very smoothly at 80 °C for 6 h to obtain desired 3-amino-2-cyano-1-(4-chlorophenyl)-1H-benzo[f] chromene in aqueous medium with maximum yield of 88% (Table 2, entry 1). When we have used acetonitrile, DMF, 1,4-dioxane or n-hexane as a solvent for the above reaction, Knoevenagel condensation adduct was formed as a major product and the desired product 3-amino-chromenes was formed only in 25%, 30%, 15% and 10% yields, respectively (Table 2, entries 3–6). But polar protic solvents such as water and ethanol have improved the yields of the desired product to 88% and 78%, respectively (Table 2, entries 1 and 2). So water is the best solvent to obtain maximum yield of 3-amino-2-cyanobenzo-chromenes over TPOP-2 organocatalyst.| Entry | Aromatic aldehyde | Product | Time (h) | Solvent | Yielda (%) | TONb |
|---|---|---|---|---|---|---|
| a All the reactions are carried out at 80 °C temperature and isolated yields of products are given. For entries 1–7 and 15 resorcinol was used as reactant whereas for entries 8–14, 2-napthol was used as reactant.b Turn over number (TON) = moles of substrate converted per mole of active site. Total basicity of the TPOP-2 material is 1.13 mmol g−1 calculated from CO2-TPD analysis.c Reaction was carried out in the presence of air. | ||||||
| 1 |  |
 |
5 | Solvent-free | 87 | 19.2 |
| 2 | 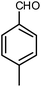 |
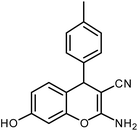 |
6 | Solvent-free | 84 | 18.6 |
| 3 | 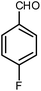 |
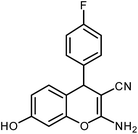 |
5 | Solvent-free | 88 | 19.5 |
| 4 | 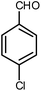 |
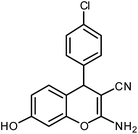 |
6 | Solvent-free | 85 | 18.8 |
| 5 | 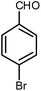 |
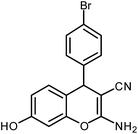 |
6 | Solvent-free | 86 | 19.0 |
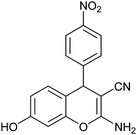
| 6 | 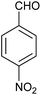 |
 |
4 | Solvent-free | 85 | 18.8 |
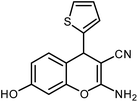
| 7 | 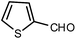 |
 |
5 | Solvent-free | 86 | 19.0 |
| 8 | 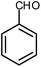 |
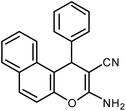 |
6 | Water | 87 | 19.2 |
| 9 | 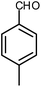 |
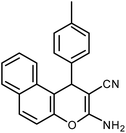 |
6 | Water | 87 | 19.2 |
| 10 | 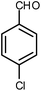 |
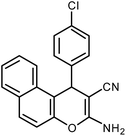 |
6 | Water | 88 | 19.5 |
| 11 | 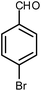 |
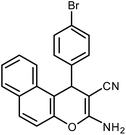 |
6 | Water | 87 | 19.2 |
| 12 | 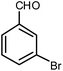 |
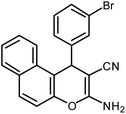 |
6 | Water | 86 | 19.0 |
| 13 | 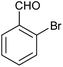 |
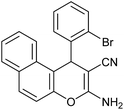 |
6 | Water | 82 | 18.1 |
| 14 | 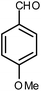 |
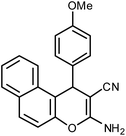 |
6 | Water | 84 | 18.6 |
| 15 | 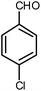 |
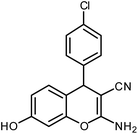 |
6 | Solvent-free | 84 | 18.3c |
| Entry | Solvent | Yieldab (%) |
|---|---|---|
| a All the reactions were carried out at 80 °C temperature except the reaction where n-hexane was used as a solvent, in case of n-hexane, the reaction temperature was 70 °C. Reaction conditions: 1 mmol 4-chlorobenzaldehyde (140 mg), 1 mmol 2-naphthol (144 mg), 1 mmol malononitrile (66 mg) and 40 mg catalyst. Solvent-free reaction was carried out at 100 °C.b Isolated yields. | ||
| 1 | Water | 88 |
| 2 | Ethanol | 78 |
| 3 | Acetonitrile | 25 |
| 4 | DMF | 30 |
| 5 | 1,4-Dioxane | 15 |
| 6 | n-Hexane | 10 |
| 7 | Solvent-free | Trace |
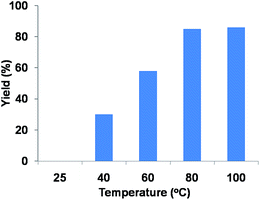
To investigate the optimum amount of organocatalyst, the unimolar reaction of 4-chlorobenzaldehyde, resorcinol and malononitrile is carried out under solvent-free conditions over different amount of TPOP-2 at 80 °C for 6 h. When the reaction was carried out at 80 °C temperature in the absence of any catalyst, very trace amount of desired product was obtained. With increasing the amount of organocatalyst from 10 mg to 40 mg, the yield of the above reaction was improved (yields are shown in Fig. 8). Further increasing of the amount of organocatalyst, there was no significant change in the yield of desired product. From Fig. 9, we can conclude that 40 mg of TPOP-2 organocatalyst is optimum to conduct these multicomponent condensation reactions.
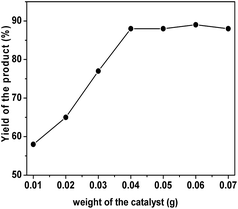 | ||
| Fig. 8 Variation of the amount of catalyst in the one pot three component condensation reaction over TPOP-2. | ||
The same condensation reaction has been carried out at different temperatures to understand the effect of temperature on the progress of the reactions. As seen in Fig. 9 that the yield of the condensation reaction for the synthesis of 2-amino-4H-chromenes over the TPOP-2 catalyst under the solvent-free condition increases with increasing the reaction temperature. Fig. 9 also suggests that 80 °C is the optimum temperature to carry out these condensation reactions in good yield. The optimized conditions for the synthesis of 2-amino-chromenes and their yields are summarized in the Table 1.
Similarly we have conducted the condensation reaction of 4-chlorobenzaldehyde, resorcinol and malononitrile using various homogeneous Lewis base such as piperidine, pyridine, imidazole and tris(2-aminoethyl)amine under optimized conditions in order to compare them with the catalytic activity of TPOP-2. Much lower yields of the products are obtained over these homogeneous base catalysts (Table 3, entries 3–6, respectively). This result suggested that TPOP-2 is an excellent heterogeneous catalyst for this 3-component coupling reaction.
| Entry | Catalyst | Temperature (°C) | Yieldab (%) |
|---|---|---|---|
| a Reaction conditions: 1 mmol 4-chlorobenzaldehyde (140 mg), 1 mmol resorcinol (110 mg), 1 mmol malononitrile (66 mg), 40 mg catalyst TPOP-2 and all the reactions are carried out for 6 h.b Isolated yields. | |||
| 1 | No catalyst | 25 | NR |
| 2 | No catalyst | 80 | Trace |
| 3 | Piperidine | 80 | 46 |
| 4 | Pyridine | 80 | 40 |
| 5 | Imidazole | 80 | 48 |
| 6 | Tris(2-aminoethyl)amine | 80 | 35 |
| 7 | TPOP-2 | 80 | 85 |
Effect of substituted groups of aromatic aldehydes
The time required for the completion of these reactions using aromatic aldehydes with electron withdrawing groups (Table 1, entry 6) were somewhat smaller than electron donating groups (Table 1, entries 2, 9 and 14). First step of the cyclization reaction is Knoevenagel condensation reaction between aromatic aldehyde and malononitrile. With increasing the electron withdrawing power of substituted groups of aromatic aldehyde, the rate of the first step gets increased. In case of heterocyclic aldehyde also the reaction underwent cleanly with excellent yield (Table 1, entry 7). On the other hand higher reactivity of the meta- and para-subtituted aromatic aldehydes (Table 1, entries 2–6, 9–12, 14) over ortho-substituted aromatic aldehydes (Table 1, entry 13) could be attributed to the steric hindrance.Plausible reaction mechanism and factors affecting the reaction
The possible reaction pathway for this three component reaction could proceed via the Knoevenagel condensation of an aromatic aldehyde and malononitrile. Then the proton of activated phenol is abstracted by N-sites of the organocatalyst and it attacks to the Knoevenagel product through Michael addition. This is followed by intramolecular cyclization to form the 2-amino-chromene derivatives.34 Since resorcinol is more acidic than 2-naphthol, the phenolic-OH proton of resorcinol can be easily abstracted. Further, due to presence of two hydroxyl groups in resorcinol, it is more electron rich and more nucleophilic than 2-naphthol. So, when we have used resorcinol as an activated phenol the reactions can proceed smoothly under solvent-free conditions with good yields. Whereas, for 2-napthol a solvent is necessary to make a better dispersion of the catalyst in the reaction medium and thus to proceed the reaction. The key factors affecting the process are solvent polarity, temperature and amount of the catalyst. In polar protic solvents, the reaction will be faster compare to non-polar solvents. Hence, water and ethanol could enhanced the rate of the reaction and gave good yields of benzochromenes.Reusability of the organocatalyst
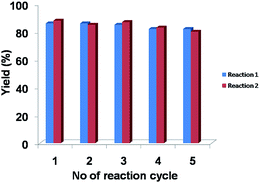
In order to check the reusability of the organocatalyst TPOP-2 two controlled condensation reactions are carried out: one condensation reaction of 4-bromobenzaldehyde, resorcinol and malononitrile under solvent-free condition and another condensation reaction of 4-chlorobenzaldehyde, 2-naphthol and malononitrile in aqueous medium. In both cases after completion of reaction, the catalyst is filtered and washed with ethyl acetate for several times and then dried at 75 °C for overnight for the next reaction. Total five consecutive cycles have been carried out in each case. In all cases TPOP-2 exhibited consistent catalytic activity (Fig. 10). From the Fig. 10 it is clear that only very little deviation in the product yield is observed in the consecutive catalytic cycles. Thus, this result suggested high catalytic efficiency of TPOP-2 organocatalyst in the one-pot three component condensation reactions.Leaching test
In order to check whether any organic group has been leached from the polymer framework of TPOP-2 or not, we have performed in situ filtration technique. In this technique, 0.04 g organocatalyst was stirred in 5 mL dichloromethane for 24 h at room temperature. Then TPOP-2 is separated by filtration and the filtrate was taken in a 50 mL round bottom flask and it is evaporated to dryness. The condensation reaction of 4-chlorobenzaldehyde, malononitrile and resorcinol is conducted similarly in a round bottom flask at 80 °C temperature for 6 h. Trace amount of desire product (same when the reaction was carried out without any catalyst) has been observed, establishing the fact that no leaching of organic group occurs. The absence of organic triazine moiety or tris(2-aminoethyl)amine group in the filtrate further confirmed by performing UV-Vis spectroscopic analysis of the filtrate, where no additional peaks corresponding to the chromophores are observed.Hot filtration test
We have carried out the condensation reaction of 4-chlorobenzaldehyde, malononitrile and resorcinol under optimized reaction condition. After 3 h the reaction has been stopped, the reaction mixture is cooled and stirred with 5 mL methanol for 30 min at room temperature. Then the catalyst is filtered and the solvent is evaporated to dryness from the reaction mixture. At this stage the yield of the desire product was 55% (confirmed by 1H NMR). After that the reaction is continued under same reaction condition for 6 h. Then the reaction is worked-up as done previously. But no increase in the product yield is observed (yield 55.2%). This result clearly confirms the heterogeneous nature of the organocatalyst TPOP-2 and no leaching of organic group from the catalyst to the reaction mixture is occurred during the course of the catalytic reaction.Characterization of the reused catalyst
We have checked wide angle powder XRD and FT IR spectroscopic data of the reused organocatalyst TPOP-2 after four consecutive catalytic cycles. In the wide angle powder XRD pattern (Fig. S1†) of TPOP-2 the characteristic broad peak for amorphous polymer is observed. FT IR spectra of the reused catalyst after fourth catalytic cycle shows two characteristic absorption bands at 1342 cm−1 and 1572 cm−1 (Fig. 4). This is attributed to the triazine rings. Further, absorption bands at 1423 and 2938 cm−1 for bending and stretching vibration of sp3 –CH2– are observed. Thus the XRD and FT IR results revealed that the framework and bonding of the porous polymer has been retained during the course of successive multicomponent coupling reactions.Conclusion
In conclusion, we have developed a new protocol to design a triazine based porous organic polymer through the unimolar reaction between cyanuric chloride and tris(2-aminoethyl)amine. This porous polymer contains abundance of basic N-sites and it has been employed as a metal-free organocatalyst for the one-pot three component condensation reaction of aromatic aldehyde, malononitrile and activated phenols (resorcinol, 2-naphthol) under solvent-free conditions (for resorcinol) or aqueous medium (for 2-naphthol) to synthesize 2-amino-chromenes. Thus, TPOP-2 organocatalyst has excellent future potential in the environment friendly liquid phase base catalyzed reactions for the synthesis of value added fine chemicals.Acknowledgements
SKK thanks CSIR, New Delhi for his senior research fellowship. AB thanks DST, New Delhi for providing instrumental facility through the DST Unit on Nanoscience, and DST-SERB and DST-UKIERI research grants.References and Notes
- (a) N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC; (b) J. Germain, J. M. J. Fréchet and F. Svec, Small, 2009, 5, 1098–1111 CrossRef CAS PubMed; (c) P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819–835 CrossRef CAS; (d) R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS PubMed.
- (a) D. C. Wu, F. Xu, B. Sun, R. W. Fu, H. K. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS PubMed; (b) J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Chem. Commun., 2014, 50, 3347–3349 RSC; (c) V. M. Suresh, S. Bonakala, H. S. Atreya, S. Balasubramanian and T. K. Maji, ACS Appl. Mater. Interfaces, 2014, 6, 4630–4637 CrossRef CAS PubMed.
- (a) M. Carta, M. Croad, K. Bugler, K. J. Msayib and N. B. McKeown, Polym. Chem., 2014, 5, 5262–5266 RSC; (b) C. Bleschke, J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Adv. Synth. Catal., 2011, 353, 3101–3106 CrossRef CAS.
- M. X. Tan, L. Gu, N. Li, J. Y. Ying and Y. Zhang, Green Chem., 2013, 15, 1127–1132 RSC.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- X. Liu, A. Sigen, Y. Zhang, X. Luo, H. Xia, H. Li and Y. Mu, RSC Adv., 2014, 4, 6447–6453 RSC.
- (a) E. Merino, E. Verde-Sesto, E. M. Maya, A. Corma, M. Iglesias and F. Sánchez, Appl. Catal., A, 2014, 469, 206–212 CrossRef CAS PubMed; (b) E. Merino, E. Verde-Sesto, E. M. Maya, M. Iglesias, F. Sánchez and A. Corma, Chem. Mater., 2013, 25, 981–988 CrossRef CAS.
- J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Chem. Commun., 2014, 50, 3347–3349 RSC.
- (a) D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602–1634 CrossRef PubMed; (b) S. Yu, X. You and Y. Liu, Chem.–Eur. J., 2012, 18, 13936–13940 CrossRef CAS PubMed; (c) L. Cala, A. Mendoza, F. J. Fañanás and F. Rodrĭguez, Chem. Commun., 2013, 49, 2715–2717 RSC.
- M. Boominathan, M. Nagaraj, S. Muthusubramanian and R. V. Krishnakumar, Tetrahedron, 2011, 67, 6057–6064 CrossRef CAS PubMed.
- N. K. Shah, N. M. Shah, M. P. Patel and R. G. Patel, J. Chem. Sci., 2013, 125, 525–530 CrossRef CAS.
- (a) L. Alvey, S. Prado, V. Huteau, B. Saint-Joanis, S. Michel, M. Koch, S. T. Cole, F. Tillequin and Y. L. Janin, Bioorg. Med. Chem., 2008, 16, 8264–8272 CrossRef CAS PubMed; (b) T. Symeonidis, M. Chamilos, D. J. Hadjipavlou-Litina, M. Kallitsakis and K. E. Litinas, Bioorg. Med. Chem. Lett., 2009, 19, 1139–1142 CrossRef CAS PubMed.
- D. R. Anderson, S. Hegde, E. Reinhard, L. Gomez, W. F. Vernier, L. Lee, S. Liu, A. Sambandam, P. A. Snidera and L. Masih, Bioorg. Med. Chem. Lett., 2005, 15, 1587–1590 CrossRef CAS PubMed.
- T. E.-S. Ali, M. A. Ibrahim and S. M. Abdel-Kariem, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2358–2392 CrossRef.
- M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295–4298 CrossRef CAS PubMed.
- (a) G. P. Ellis, in Chemistry of Heterocyclic Compounds Chromenes, Chromanes and Chromones, ed. A. Weissberger and E. C. Taylor, John Wiley, New York, 1977, ch. II, pp. 11–139 Search PubMed; (b) E. A. A. Hafez, M. H. Elnagdi, A. G. A. Elagamey and F. M. A. A. El-Taweel, Heterocycles, 1987, 26, 903–907 CrossRef CAS.
- Y. Peng and G. Song, Catal. Commun., 2007, 8, 111–114 CrossRef CAS PubMed.
- O. Rosati, M. Curini, M. C. Marcotullio, G. Oball-Mond, C. Pelucchini and A. Procopio, Synthesis, 2010, 239–248 CrossRef CAS PubMed.
- A.-Q. Zhang, M. Zhang, H.-H. Chen, J. Chen and H.-Y. Chen, Synth. Commun., 2007, 37, 231–235 CrossRef CAS.
- Y. Ren and C. Cai, Catal. Commun., 2008, 9, 1017–1020 CrossRef CAS PubMed.
- B. S. Kumar, N. Srinivasulu, R. H. Udupi, B. Rajitha, Y. T. Reddy, P. N. Reddy and P. S. Kumar, J. Heterocycl. Chem., 2006, 43, 1691–1693 CrossRef CAS.
- N. V. Lakshmi, S. E. Kiruthika and P. T. Perumal, Synlett, 2011, 1389–1394 CAS.
- A. Shaabani, R. Ghadari, S. Ghasemi, M. Pedarpour, A. H. Rezayan, A. Sarvary and S. Weng Ng, J. Comb. Chem., 2009, 11, 956–959 CrossRef CAS PubMed.
- R. M. N. Kalla, S. J. Byeon and M. S. H. Kim, Tetrahedron, 2013, 69, 10544–10551 CrossRef CAS PubMed.
- M. M. Heravi, B. Baghernejad and H. A. Oskooie, J. Chin. Chem. Soc., 2008, 55, 659–662 CAS.
- R. Ballini, G. Bosica, M. L. Conforti, R. Maggi, A. Mazzacani, P. Righi and G. Sartori, Tetrahedron, 2001, 57, 1395–1398 CrossRef CAS.
- T. S. Jin, J. C. Xiao, S. J. Wang, T. S. Li and X. R. Song, Synlett, 2003, 2001–2004 CrossRef CAS.
- L. Chen, Y. Q. Li, X. J. Huang and W. J. Zheng, Heteroat. Chem., 2009, 20, 91–94 CrossRef CAS.
- S. K. Kundu, J. Mondal and A. Bhaumik, Dalton Trans., 2013, 10515–10524 RSC.
- D. Kumar, V. B. Reddy, B. G. Mishra, R. K. Rana, M. N. Nadagaouda and R. S. Varma, Tetrahedron, 2007, 63, 3093–3097 CrossRef CAS PubMed.
- M. M. Heravi, K. Bakhtiari, V. Zadsirjan and F. F. Bamoharram, Bioorg. Med. Chem. Lett., 2007, 17, 4262–4265 CrossRef CAS PubMed.
- R. Maggi, R. Ballini, G. Sartori and R. Sartorio, Tetrahedron Lett., 2004, 45, 2297–2299 CrossRef CAS PubMed.
- P. C. B. Page, A. Mace, D. Arquier, D. Bethell, B. R. Buckley, D. J. Willock and G. J. Hutchings, Catal. Sci. Technol., 2013, 3, 2330–2339 Search PubMed.
- J. Mondal, A. Modak, M. Nandi, H. Uyama and A. Bhaumik, RSC Adv., 2012, 2, 11306–11317 RSC.
- (a) M. Pramanik, M. Nandi, H. Uyama and A. Bhaumik, Catal. Sci. Technol., 2012, 2, 613–620 RSC; (b) V. Kumari, A. K. Patra and A. Bhaumik, RSC Adv., 2014, 4, 13626–13634 RSC.
- M. J. Bojdys, J. Jeromenok, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 2202–2205 CrossRef CAS PubMed.
- P. Puthiaraja and K. Pitchumani, Green Chem., 2014, 16, 4223–4233 RSC.
- V. Hankova, E. Slovakova, J. Zednik, J. Vohlidal, R. Sivkova, H. Balcar, A. Zukal, J. Brus and J. Sedlacek, Macromol. Rapid Commun., 2012, 33, 158–163 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00951k |
| This journal is © The Royal Society of Chemistry 2015 |