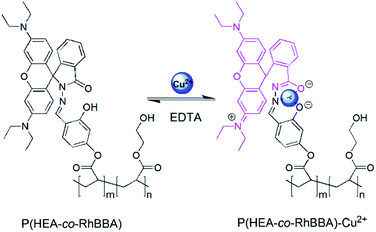
A novel reversible colorimetric chemosensor for the detection of Cu2+ based on a water-soluble polymer containing rhodamine receptor pendants†
Guang Li*a,
Farong Taoa,
Hu Wangb,
Liping Wanga,
Jiaojiao Zhanga,
Peipei Gea,
Lin Liua,
Yunhua Tonga and
Su Suna
aSchool of Materials Science and Engineering, Liaocheng University, Liaocheng 252059, China. E-mail: lglzsd@126.com; Fax: +86 635 8230831; Tel: +86 635 8230919
bCenter of Analysis and Testing of China National Metrology Accredited Laboratory, Anhui University, Hefei 230039, China
First published on 9th February 2015
Abstract
A novel reversible colorimetric chemosensor based on a water-soluble polymer containing rhodamine receptor pendants has been developed. The chemosensor exhibited high sensitivity and selectivity to Cu2+ ions with a short response time in pure aqueous solution, and the results could be monitored directly by the naked eye. The detection limit was 3.6 × 10−7 M with UV-vis absorbance experiment. The absorption intensity of P(HEA-co-RhBBA) for Cu2+ remained unaffected over a wide pH range of 4–10. In addition, low-cost test strips were fabricated using P(HEA-co-RhBBA) for convenient and efficient detection of Cu2+ ions.
Introduction
As one of the most important transition metal ions, copper ions play a crucial role in the life of organisms. However, unregulated overloading of copper causes toxicity and can result in severe neurodegenerative diseases such as Alzheimer's, Wilson's and Parkinson's diseases.1 Therefore, highly sensitive and selective detection of Cu2+ ions in water is important for human health and environmental protection. During the last couple of decades, considerable attention has been paid to the design and synthesis of Cu2+ ion chemosensors, including colorimetric chemosensors,2 fluorescent chemosensors3 and electrochemical sensors.4 Among them, colorimetric chemosensors based on color changes are more attractive and convenient since they can be monitored directly by the naked eye and avoid using complex instruments.Since the first report about the detection of Cu2+ ions using rhodamine B derivative in 1997,3a rhodamine derivatives have been considered as ideal candidates for constructing chemosensors due to their excellent spectroscopic properties, and a great deal of small molecule rhodamine derivatives have been synthesized to detect various metal ions.5 However, owing to the poor water solubility of the small molecule rhodamine derivatives with spirolactam ring, organic solvents must be used in the process of metal ions detection, and this partially limits their potential applications in the environmental and biological fields. Furthermore, the design of chemosensors for practical application is still a challenge because of the limitations of small molecules in the fabrication of devices. In contrast to small molecular chemosensors, chemosensors based on polymers can be easily employed to fabricate devices.6 Recently, Wu et al.7 reported a water-soluble conjugated polymer bearing rhodamine 6G moieties, which showed high selectivity and good reversibility toward Fe3+ in pure aqueous solution. Covalent incorporation of ion-sensitive receptors into hydrophilic polymeric backbones paves a way to develop novel water-soluble chemosensors, and avoids the second pollution from the use of organic cosolvents in the detection process of metal ions. However, to the best of our knowledge, the reports about rhodamine-based polymers as chemosensors to real-time monitor of Cu2+ ions in pure aqueous solution are quite limited.
Herein, we report a novel, reversible water-soluble polymer chemosensor, P(HEA-co-RhBBA), which contains the pendant group of rhodamine B-based dye as the ion-sensitive receptor. Intriguingly, not only does P(HEA-co-RhBBA) exhibit rapid selective response to Cu2+ ions in pure aqueous solution with turn-on color, but also the relative metal complex, P(HEA-co-RhBBA)–Cu2+, shows turn-off color with high sensitivity to EDTA ions. In order to detect Cu2+ ions conveniently, the test strips were prepared using P(HEA-co-RhBBA).
Experimental
Material and methods
Azobisisobutyronitrile (AIBN, Shanghai Chemical Reagent Co., China) was recrystallized from ethanol. Rhodamine B, hydrazine hydrate, 2,4-dihydroxybenzaldehyde, 2-hydroxyethyl acrylate (HEA), nitrate salts of metal ions (Ba2+, Co2+, Cd2+, Cr3+, Fe3+, Hg2+, Mn2+, Na+, Pb2+, Zn2+), chloride salt of Fe2+ and all other chemicals were purchased from Shanghai Chemical Reagent Co. Deionized water was used throughout the experiment. Rhodamine B hydrazide,8 acryloyl chloride,9 and benzyl 1H-imidazole-1-carbodithioate (BICDT)10 were synthesized according to the references, respectively. The normal filter paper was used to prepare the test strip and purchased from Hangzhou Special Paper Industry Co.The values of number-average molecular weight (Mn) and polydispersity index (PDI) were determined by means of a waters 150C gel permeation chromatograph (GPC) equipped with 103, 104, and 105 Å waters ultrastyragel columns, using DMF (1.0 mL min−1) as the eluent, and the calibration was carried out with a polystyrene standard. NMR spectra were recorded at room temperature on a Varian Mercury 400 spectrometer (at 400 MHz for 1H NMR and 100 MHz for 13C NMR) using CDCl3 as the solvent and TMS as the internal standard. High resolution mass spectra (HRMS) were measured on a Thermo Fisher Scientific LTQ Orbitrap XL. UV-vis absorption spectra were measured using a Shimadzu UV-3600 spectrophotometer. Fluorescent spectra were recorded on a Hitachi F-7000 fluorescence spectrophotometer. The pH values were measured on a Mettler-Toledo FE20 pH meter.
Synthesis of compound RhBHB
Under nitrogen, rhodamine B hydrazide (0.46 g, 1 mmol) and 2,4-dihydroxybenzaldehyde (0.276 g, 2 mmol) were combined in ethanol (20 mL) and refluxed for 8 h. After that, the solution was cooled and allowed to stand at room temperature overnight. The precipitate was filtered and washed 3 times with 10 mL cold ethanol. After drying under reduced pressure, RhBHB was obtained as a light pink powder (0.41 g, yield: 72%). 1H-NMR (400 MHz, CDCl3), δ (ppm): 1.14 (t, 12H, J = 7 Hz), 3.32 (q, 8H, J = 7.2 Hz), 6.28 (dd, 2H, J = 8.8 Hz, J = 2.4 Hz), 6.31 (dd, 1H, J = 8.8 Hz, J = 2.4 Hz), 6.35 (d, 1H, J = 2.4 Hz), 6.47 (d, 2H, J = 2.4 Hz), 6.50 (d, 2H, J = 8.8 Hz), 6.96 (d, 1H, J = 8.4 Hz), 7.18 (dd, 1H, J = 6.4 Hz, J = 1.6 Hz), 7.51 (m, 2H), 7.97 (dd, 1H, J = 6.8 Hz, J = 1.6 Hz), 9.19 (s, 1H), 11.02 (s, 2H); 13C-NMR (100 MHz, CDCl3), δ (ppm): 12.6, 44.3, 66.7, 98.1, 103.5, 105.6, 107.2, 108.2, 112.3, 123.2, 124.1, 128.1, 128.5, 130.2, 133.1, 133.3, 149.1, 150.6, 153.6, 154.1, 159.0, 160.7, 164.1; HRMS (ESI) calcd for C35H36N4O4 [M + H]+ (m/z): 577.2770; found 577.2795.Synthesis of monomer RhBBA
A solution of RhBHB (0.1 g, 0.17 mmol) in dry CH2Cl2 (3 mL) was added triethylamine (21.1 mg, 0.21 mmol) at 0 °C, and the mixture was stirred for 5 min. Then, a solution of acryloyl chloride (15.7 mg, 0.17 mmol) in dry CH2Cl2 (3 mL) was added dropwise over 15 min. The reaction mixture was allowed to warm to room temperature, and stirred overnight. After the reaction, the mixture was poured into 15 mL CH2Cl2 and washed with water (5 × 25 mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified with a column chromatography on silica gel (CH2Cl2/MeOH = 10/1) and a light pink powder was collected (85.8 mg, yield: 80%). 1H-NMR (400 MHz, CDCl3), δ (ppm): 1.16 (t, 12H, J = 7 Hz), 3.33 (q, 8H, J = 7.2 Hz), 6.00 (dd, 1H, J = 10.4 Hz, J = 0.8 Hz), 6.27 (dd, 2H, J = 8.4 Hz, J = 2.4 Hz), 6.30 (m, 1H), 6.47 (d, 2H, J = 2.8 Hz), 6.50 (d, 2H, J = 8.8 Hz), 6.58 (dd, 1H, J = 17.2 Hz, J = 0.8 Hz), 6.59 (dd, 1H, J = 8.8 Hz, J = 2.4 Hz), 6.64 (d, 1H, J = 2.4 Hz), 7.11 (d, 1H, J = 8.4 Hz), 7.19 (dd, 1H, J = 6.4 Hz, J = 1.6 Hz), 7.53 (m, 2H), 7.98 (dd, 1H, J = 6.8 Hz, J = 1.6 Hz), 9.17 (s, 1H), 11.11 (s, 1H); 13C-NMR (100 MHz, CDCl3), δ (ppm): 12.6, 44.3, 45.8, 66.4, 97.9, 105.2, 108.1, 112.4, 116.7, 123.3, 124.1, 127.7, 128.0, 128.5, 129.9, 132.1, 132.6, 133.4, 149.1, 150.7, 151.8, 152.6, 153.5, 159.7, 164.2; HRMS (ESI) calcd for C38H38N4O5 [M + H]+ (m/z): 631.2876; found 631.2891.Synthesis of P(HEA-co-RhBBA)
BICDT (15.6 mg, 66.7 μmol), AIBN (2.7 mg, 16.7 μmol), RhBBA (52.0 mg, 82.5 μmol), HEA (0.948 g, 8.16 mmol) and 2-propanol (1 mL) were placed in a glass tube. The glass tube was sealed under vacuum after degassing with three freeze–evacuate–thaw cycles and then placed in an oil bath at 65 °C. After the polymerization was conducted for 2 h, the reaction mixture was cooled to room temperature and precipitated in ethyl acetate for 3 times. The polymer was collected by filtration and dried in a vacuum oven at room temperature for 24 h to give a slightly pink solid (0.72 g, Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, PDI = 1.22).
500, PDI = 1.22).
Results and discussion
Synthesis
As shown in Scheme 1, a novel polymerizable Cu2+ receptor monomer, RhBBA, which was also an example of the rhodamine spirolactam family, was firstly synthesized by the reaction of RhBHB with acryloyl chloride. The structures of RhBHB and RhBBA were characterized by 1H NMR, 13C NMR, and HRMS analyses (Fig. S1–S6, ESI†). To overcome the poor water solubility of the small molecule rhodamine derivatives with spirolactam ring, 2-hydroxyethyl acrylate (HEA) as a neutral hydrophilic chain segment was chosen as comonomer to form the water-soluble polymer matrix. And then, well-defined water-soluble polymers P(HEA-co-RhBBA) were facilely synthesized via reversible addition-fragmentation chain transfer (RAFT) polymerization (Scheme 1). RhBBA content in the P(HEA-co-RhBBA) chain was determined to be ∼0.9 mol% from the standard working curve in UV-vis absorption spectra. Compared to other small molecule rhodamine derivatives with spirolactam ring, P(HEA-co-RhBBA) exhibits excellent solubility in pure aqueous solution without addition of any organic cosolvent.Spectroscopic properties
High selectivity is usually required for a chemosensor to accomplish detection successfully. In order to verify the selectivity of the synthesized chemosensor, a variety of metal ions (Na+, Ba2+, Co2+, Cd2+, Cr3+, Cu2+, Fe2+, Fe3+, Mn2+, Zn2+, Hg2+, and Pb2+) were tested. Like most of the spirolactam rhodamine derivatives, the free P(HEA-co-RhBBA) (10 μM RhBBA) remained colorless and did not exhibit apparent absorption above 500 nm in an aqueous solution (Fig. 1), which indicated that the spirolactam form was the predominant species in the pendant group of rhodamine B-based dyes. Upon addition of 5 equiv. of Cu2+ ions, a strong absorption band centered at 561 nm was observed and led to the color change of the solution from colorless to purple (Fig. 1a). In contrast, addition of 5 equiv. of other metal ions led to no obvious color change and spectral enhancements (Fig. 1).To check the applicability of P(HEA-co-RhBBA) as a Cu2+ selective colorimetric chemosensor, we carried out competitive experiments in the presence of 5 equiv. of Cu2+ ions mixed with Na+, Ba2+, Co2+, Cd2+, Cr3+, Fe2+, Fe3+, Mn2+, Zn2+, Hg2+, and Pb2+ (50 equiv. each). Results are shown in Fig. 2, and no significant variation in the absorbance is observed by comparison with or without other metal ions. These results demonstrate that the response of the chemosensor to Cu2+ ions is unaffected by the presence of other metal ions, even existing in a concentration 10 times higher than that of Cu2+ ions. It is well known that most of rhodamine-based chemosensors show the fluorescence enhancement effect upon addition of analytes.3 However, after coordination with Cu2+, P(HEA-co-RhBBA) only exhibited the strong absorption and the characteristic color change, and did not generate the strong fluorescence emission of rhodamine moiety (Fig. S7, ESI†). It can be attributed to the quenching property of paramagnetic Cu2+. Similar phenomena were also observed in other studies.11 Addition of 5 equiv. of other surveyed metal ions (Na+, Ba2+, Co2+, Cd2+, Cr3+, Fe2+, Fe3+, Mn2+, Zn2+, Hg2+, and Pb2+, respectively) also did not produce a distinct increase in the emission intensity of P(HEA-co-RhBBA) (Fig. S7, ESI†).
UV-vis absorbance titration of P(HEA-co-RhBBA) (10 μM RhBBA) with Cu2+ ions was conducted in an aqueous solution. Upon addition of increasing concentrations of Cu2+ ions, the color of the aqueous solution changed from colorless to purple gradually (Fig. S8, ESI†), and the absorbance intensity at 561 nm increased obviously (Fig. 3). Until the concentration of Cu2+ ions increased to 1.5 equiv., the saturation of the absorbance intensity was achieved (Fig. 3, inset). According to the linear Benesi–Hildebrand expression, the measured absorbance [1/(A − A0)] at 561 nm varied as a function of 1/[Cu2+] in a linear relationship with a correlation coefficient of 0.9927 (Fig. S9†), and the binding constant of P(HEA-co-RhBBA) with the Cu2+ ion was calculated to be 2.76 × 105 M−1. The detection limit of P(HEA-co-RhBBA) as a colorimetric chemosensor for the analysis of Cu2+ ions was found to be 3.6 × 10−7 M (Fig. S10, ESI†),12 which was lower than some of the reported detection limits.2c–e,5h The U.S. Environmental Protection Agency (EPA) has set the safe limit of Cu2+ in drinking water at 20 μM.13 Hence, P(HEA-co-RhBBA) can be a powerful tool for the detection of Cu2+ ions in drinking water.
Besides high sensitivity and selectivity, a short response time is also an important factor for a colorimetric chemosensor to monitor analytes in practical analysis. And so, the response time of P(HEA-co-RhBBA) to 2 equiv. of Cu2+ ions in aqueous solution was investigated. The experimental results showed that the color change of the solution completed within 10 s after addition of Cu2+ ions (movie S1, ESI†), which meant the high-speed response of P(HEA-co-RhBBA)-based colorimetric chemosensor to Cu2+ ions. Therefore, the colorimetric chemosensor can be used for the real-time monitor of Cu2+ ions.
For the efficient monitor of Cu2+ ions in environmental samples, the absorption spectral changes of P(HEA-co-RhBBA) should take place over a wide pH range. Thus, the effect of pH on the absorption response of P(HEA-co-RhBBA) in the absence and presence of Cu2+ ions was investigated in aqueous solution with the pH range of 2 to 13. As shown in Fig. 4, nearly no obvious change in the absorption band of P(HEA-co-RhBBA) at 561 nm is observed in the pH range from 2 to 13, indicating that the free P(HEA-co-RhBBA) is stable in the wide pH range. Upon the addition of Cu2+ ions, absorption intensity at 561 nm increases obviously, meaning the formation of P(HEA-co-RhBBA)–Cu2+ complex. The absorption band of the complex at 561 nm remains unaffected in solution with the pH value between 4 and 10. Thus, the uniform activity over such a wide range of pH makes P(HEA-co-RhBBA) suitable for the analysis of environmental samples that would occur well within this extended range of pH, or for use in unbuffered medium.
 | ||
| Fig. 4 Effect of pH on the absorbance intensity of P(HEA-co-RhBBA) in aqueous solutions (10 μM RhBBA) in the absence (a) and presence (b) of Cu2+ ions (5 equiv.). | ||
Reversibility studies
In order to achieve the reversibility of the chemosensor, the color and absorbance of P(HEA-co-RhBBA)–Cu2+ complex should be successfully recovered through chelating of Cu2+ to another competing ligand with stronger binding force. As we know, Cu2+ ion can interact with EDTA, which is a hexadentate chelating ligand, to form an octahedral complex. Compared with P(HEA-co-RhBBA), EDTA has stronger binding capability with Cu2+, and can capture Cu2+ from P(HEA-co-RhBBA)–Cu2+ complex easily and transform into a more stable EDTA–Cu2+ complex. As shown in Fig. S11 (ESI†), a new absorption peak appears upon addition of Cu2+ ions to P(HEA-co-RhBBA) aqueous solution, and the original absorption of P(HEA-co-RhBBA) can recover after addition of EDTA to P(HEA-co-RhBBA)–Cu2+ complex solution. Obviously, the absorption of recovered P(HEA-co-RhBBA) can appear again by further adding Cu2+ ions, and the absorption of P(HEA-co-RhBBA) can be restored steadily upon repeated addition of EDTA. As can be seen from Fig. 5b, the P(HEA-co-RhBBA)-based colorimetric chemosensor shows excellent stability with no significant absorbance signal decay for several successive cycles, which is important for the practical applications of the chemosensors. A reversible chemosensing example of P(HEA-co-RhBBA) was presented in movie S2 (ESI†), which demonstrated an on–off–on color switching process upon adding Cu2+ and EDTA ions alternately.Proposed mechanism
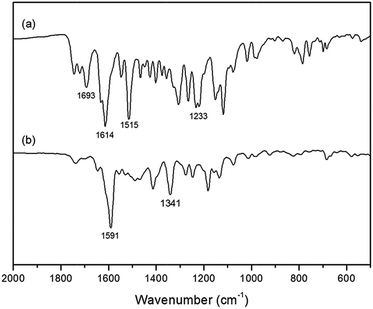
The proposed binding mechanism of P(HEA-co-RhBBA) with Cu2+ is shown in Scheme 2, which is the same as other spirolactam rhodamine derivatives.14 The solution of P(HEA-co-RhBBA) was colorless and exhibited no apparent absorption above 500 nm because RhBBA existed in the closed spirolactam form. However, upon the addition of Cu2+ ions into P(HEA-co-RhBBA) solution, the coordination of Cu2+ with carbonyl O, imino N, and phenol O atoms of RhBBA transformed the spirolactam ring into its open-ring form, resulting in color change and strong absorption band appearance. The above-mentioned reversibility experiment can serve as experimental evidence to support this reversible spirolactam ring-opening mechanism.To confirm the proposed mechanism, the FT-IR spectra experiments were carried out. As demonstrated in Fig. 6a, the stretching vibration absorption peaks of spirolactam C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and phenol C–OH appear at 1693 cm−1, 1515 cm−1 and 1233 cm−1, respectively. When RhBBA coordinates with Cu2+, the stretching vibration absorption peaks of spirolactam C
N and phenol C–OH appear at 1693 cm−1, 1515 cm−1 and 1233 cm−1, respectively. When RhBBA coordinates with Cu2+, the stretching vibration absorption peaks of spirolactam C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N disappear, and the stretching vibration absorption peak of phenol C–O shifts to higher wavenumber at 1341 cm−1 (Fig. 6b). It can be concluded that Cu2+ coordinates with carbonyl O, imino N, and phenol O atoms of RhBBA. The proposed mechanism can be further confirmed by the Job's plot (Fig. 7). The total concentration of RhBBA and Cu2+ ions was kept unchanged at 10 μM, while the concentration of Cu2+ ions increased from 0 μM to 10 μM. When the molar fraction of Cu2+ ions is 0.5, the absorbance intensity at 561 nm goes through a maximum, which implies that a 1
N disappear, and the stretching vibration absorption peak of phenol C–O shifts to higher wavenumber at 1341 cm−1 (Fig. 6b). It can be concluded that Cu2+ coordinates with carbonyl O, imino N, and phenol O atoms of RhBBA. The proposed mechanism can be further confirmed by the Job's plot (Fig. 7). The total concentration of RhBBA and Cu2+ ions was kept unchanged at 10 μM, while the concentration of Cu2+ ions increased from 0 μM to 10 μM. When the molar fraction of Cu2+ ions is 0.5, the absorbance intensity at 561 nm goes through a maximum, which implies that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry is the most possible binding mode of RhBBA and Cu2+. More direct evidence for the formation of a 1
1 stoichiometry is the most possible binding mode of RhBBA and Cu2+. More direct evidence for the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was obtained by comparing the HRMS spectra of RhBBA and RhBBA–Cu2+ (Fig. S6 and S12, ESI†). The unique peak at m/z = 692.2518 corresponding to [RhBBA + Cu–H]+ was clearly observed when 1.5 equiv. of Cu2+ was added to RhBBA.
1 complex was obtained by comparing the HRMS spectra of RhBBA and RhBBA–Cu2+ (Fig. S6 and S12, ESI†). The unique peak at m/z = 692.2518 corresponding to [RhBBA + Cu–H]+ was clearly observed when 1.5 equiv. of Cu2+ was added to RhBBA.
Applications
To investigate the practical application of the colorimetric chemosensor, test strips were facilely prepared by immersing the normal filter papers into a methanol solution of P(HEA-co-RhBBA) (10 mg mL−1) and then drying in a vacuum oven. The test strips were utilized to sense Cu2+ and other metal ions. As shown in Fig. 8a, when the test strip is dipped in 10−7 M aqueous solution of Cu2+ ions, the color change of the test strip from white to light pink is observed. The color of the test strip changes to purple immediately after being immersed into 10−4 M Cu2+ solution. Furthermore, potentially competitive metal ions have no influence on the detection of Cu2+ ions by the test strips (Fig. 8b). The feasibility of the test strips for practical applications was investigated using Cu2+ samples (10−4 M) in tap water and river water (Tuhai river). As can be seen from Fig. S13 (ESI†), the color of the test strips all change from white to purple immediately after being immersed into the different Cu2+ solutions. Therefore, the test strips can conveniently detect Cu2+ ions in pure aqueous solutions.Conclusions
In summary, we have reported a novel water-soluble polymer P(HEA-co-RhBBA) as the highly selective and sensitive colorimetric chemosensor, which can specifically detect Cu2+ ions with a short response time in pure aqueous solution by naked eyes and UV-vis absorption responses. Other competitive ions have nearly no influence on the probing behaviour. The detection limit of P(HEA-co-RhBBA) with Cu2+ was 3.6 × 10−7 M. The absorption intensity of P(HEA-co-RhBBA)–Cu2+ complex remained unaffected over a wide pH range of 4–10 and the use of P(HEA-co-RhBBA) to detect Cu2+ ions could be applied to analyse environmental samples over the wide pH range. In addition, the response toward Cu2+ ions has been established to be reversible by the EDTA-titration experiments. Furthermore, test strips based on P(HEA-co-RhBBA) have been fabricated, which can serve as a practical colorimetric sensor for the detection of Cu2+ ions through naked eye inspection with no requirement of other instruments. The present study demonstrates that water-soluble polymer containing pendant ion-sensitive receptors can be a promising platform for the detection of metal ions in pure aqueous solutions.Acknowledgements
This work was supported by the Scientific Research Award Fund for Excellent Middle-Aged and Young Scientists of Shandong Province (no. 2012CL009, 2011CL011), and the Natural Science Foundation of China (no. 21203085).Notes and references
- (a) G. Multhaup, A. Schlicksupp, L. Hesse, D. Beher, T. Ruppert, C. L. Masters and K. Beyreuther, Science, 1996, 271, 1406 CAS; (b) C. Deraeve, C. Boldron, A. Maraval, H. Mazarguil, H. Gornitzka, L. Vendier, M. Pitie and B. Meunier, Chem.–Eur. J., 2008, 14, 682 CrossRef CAS PubMed; (c) L. I. Bruijn, T. M. Miller and D. W. Cleveland, Annu. Rev. Neurosci., 2004, 27, 723 CrossRef CAS PubMed.
- (a) T. Gunnlaugsson, J. P. Leonard and N. S. Murray, Org. Lett., 2004, 6, 1557 CrossRef CAS PubMed; (b) Q. Xu, K. M. Lee, F. Wang and J. Yoon, J. Mater. Chem., 2011, 21, 15214 RSC; (c) V. Chandrasekhar, S. Das, R. Yadav, S. Hossain, R. Parihar, G. Subramaniam and P. Sen, Inorg. Chem., 2012, 51, 8664 CrossRef CAS PubMed; (d) J. Y. Noh, G. J. Park, Y. J. Na, H. Y. Jo, S. A. Lee and C. Kim, Dalton Trans., 2014, 43, 5652 RSC; (e) U. N. Yadav, P. Pant, S. K. Sahoo and G. S. Shankarling, RSC Adv., 2014, 4, 42647 RSC; (f) H. Kim, Y. J. Na, E. J. Song, K. B. Kim, J. M. Bae and C. Kim, RSC Adv., 2014, 4, 22463 RSC.
- (a) V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386 CrossRef CAS; (b) H. S. Jung, P. S. Kwon, J. W. Lee, J. Il Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS PubMed; (c) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed; (d) X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590 CrossRef CAS PubMed; (e) V. Luxami, A. S. Gupta and K. Paul, New J. Chem., 2014, 38, 2841 RSC; (f) X. Yang, W. Zeng, L. Wang, X. Lu, Y. Yan, J. Qu and R. Liu, RSC Adv., 2014, 4, 22613 RSC.
- (a) B. K. Jena and C. R. Raj, Anal. Chem., 2008, 80, 4836 CrossRef CAS PubMed; (b) Y. Wei, C. Gao, F. L. Meng, H. H. Li, L. Wang, J. H. Liu and X. J. Huang, J. Phys. Chem. C, 2012, 116, 1034 CrossRef CAS; (c) X. C. Fu, J. Wu, J. Li, C. G. Xie, Y. S. Liu, Y. Zhong and J. H. Liu, Sens. Actuators, B, 2013, 182, 382 CrossRef CAS PubMed; (d) J. Yan, K. Wang, Q. Liu, J. Qian, X. Dong, W. Liu and B. Qiu, RSC Adv., 2013, 3, 14451 RSC; (e) H. Xu, J. Yan, X. She, L. Xu, J. Xia, Y. Xu, Y. Song, L. Huang and H. Li, Nanoscale, 2014, 6, 1406 RSC.
- (a) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC; (b) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed; (c) M. Formica, V. Fusi, L. Giorgi and M. Micheloni, Coord. Chem. Rev., 2012, 256, 170 CrossRef CAS PubMed; (d) H. Zheng, X. Q. Zhan, Q. N. Bian and X. J. Zhang, Chem. Commun., 2013, 49, 429 RSC; (e) X. Zhang, X. Huang and Z. Zhu, RSC Adv., 2013, 3, 24891 RSC; (f) M. J. Culzoni, A. Muñoz de la Peña, A. Machuca, H. C. Goicoechea and R. Babiano, Anal. Methods, 2013, 5, 30 RSC; (g) K. Bera, A. K. Das, M. Nag and S. Basak, Anal. Chem., 2014, 86, 2740 CrossRef CAS PubMed; (h) S. Goswami, S. Maity, A. C. Maity, A. K. Maity, A. K. Das and P. Saha, RSC Adv., 2014, 4, 6300 RSC; (i) Y. Zhu, X. Zhang, H. Guo and Z. Zhu, RSC Adv., 2014, 4, 45791 RSC; (j) K. Wu, H. Xiao, L. Wang, G. Yin, Y. Quana and R. Wang, RSC Adv., 2014, 4, 39984 RSC; (k) J. Cui, D. Li, S. Shen, J. Liu and B. Zhao, RSC Adv., 2015, 5, 3875 RSC.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79 RSC.
- Y. Wu, J. Li, L. Liang, D. Lu, J. Zhang, G. Mao, L. Zhou, X. Zhang, W. Tan, G. Shen and R. Yu, Chem. Commun., 2014, 50, 2040 RSC.
- Y. Xiang, A. Tong, P. Jin and J. Yong, Org. Lett., 2006, 8, 2863 CrossRef CAS PubMed.
- G. H. Stempel Jr, R. P. Cross and R. P. Mariella, J. Am. Chem. Soc., 1950, 72, 2299 CrossRef.
- D. B. Hua, R. K. Bai, W. Q. Lu and C. Y. Pan, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5670 CrossRef CAS.
- (a) J. Hu, L. Dai and S. Liu, Macromolecules, 2011, 44, 4699 CrossRef CAS; (b) L. F. Zhang, J. L. Zhao, X. Zeng, L. Mu, X. K. Jiang, M. Deng, J. X. Zhang and G. Wei, Sens. Actuators, B, 2011, 160, 662 CrossRef CAS PubMed; (c) H. Y. Lee, K. M. K. Swamya, J. Y. Jung, G. Kim and J. Yoon, Sens. Actuators, B, 2013, 182, 530 CrossRef CAS PubMed; (d) Y. Yang, C. Gao, B. Li, L. Xu and L. Duan, Sens. Actuators, B, 2014, 199, 121 CrossRef CAS PubMed.
- G. J. Park, Y. J. Na, H. Y. Jo, S. A. Lee and C. Kim, Dalton Trans., 2014, 43, 6618 RSC.
- World Health Organization, Guidelines for drinking-water quality, Geneva, 1996 Search PubMed.
- Z. Xu, L. Zhang, R. Guo, T. Xiang, C. Wu, Z. Zheng and F. Yang, Sens. Actuators, B, 2011, 156, 546 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures and movies. See DOI: 10.1039/c5ra00745c |
| This journal is © The Royal Society of Chemistry 2015 |