DNA-capped Fe3O4/SiO2 magnetic mesoporous silica nanoparticles for potential controlled drug release and hyperthermia
Abstract
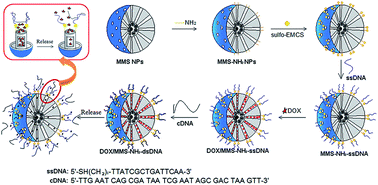
We proposed a strategy to construct DNA-capped Fe3O4/SiO2 magnetic mesoporous silica (MMS) nanoparticles for potential temperature controlled drug release and magnetic hyperthermia. Drug release behavior, magnetic heating capacity, in vitro cytotoxicity, and cell uptake of the MMS-based nanocarriers were evaluated. The results showed that the DOX/MMS–NH2–dsDNA complexes could release DOX fast at 50 °C, but very slow at 37 °C. Also, MMS-based nanocarriers could efficiently generate heat upon exposure to an alternating magnetic field due to the superparamagnetic behavior. Furthermore, the MMS–NH2–dsDNA complexes could be effectively taken up by murine breast cancer 4T1 cells, and negligible cytotoxicity of the MMS–NH2–dsDNA complexes has been observed. Therefore, DNA-capped MMS nanoparticles had potential for cancer therapy with temperature controlled drug release and magnetic hyperthermia.

 Please wait while we load your content...
Please wait while we load your content...