Fluorescence chemosensors based on functionalized SBA-15 for detection of Pb2+ in aqueous media†
Liyan Zhaoa,
Dan Suib and
Yan Wang*a
aAcademy of Fundamental and Interdisciplinary Science, Harbin Institute of Technology, Harbin 150001, China. E-mail: wangy_msn@hit.edu.cn; Tel: +86-451-86403719
bManagement Office of Laboratory and Equipment (Center of Analysis and Testing), Northeast Forestry University, Harbin 150040, China
First published on 30th January 2015
Abstract
A highly ordered mesoporous silica material (SBA-15) functionalized with 5-(4-carboxy-phenylazo)-8-hydroxyquinoline (CPA-8-HQL) for use as a fluorescence chemosensor for Pb2+ detection has been reported in this study. XRD, TEM, FT-IR, UV-vis diffuse reflectance spectra and TGA were used to characterize the grafting process. The results proved that CPA-8-HQL was successfully anchored into the channel of SBA-15 and that the primary ordered mesoporous structure of SBA-15 was well preserved. A fluorescence spectrophotometer was utilized to investigate the sensing properties, and a highly fluorescent enhancement at 429 nm was observed in the presence of Pb2+. Measurements of the sensitivity parameters demonstrated that the obtained organic/inorganic hybrid possessed excellent sensitivity and selectivity to Pb2+ in aqueous media. The lowest limit of detection was 4.90 × 10−7 mol L−1 for Pb2+.
Introduction
Some heavy metal ions, such as Fe3+, Zn2+, Cu2+ and Co2+, are essential for the maintenance of the human metabolism. However, high concentrations of these ions can lead to many adverse health effects.1–5 Hg2+, Cd2+ and Pb2+ are among the most toxic ions known. The accumulation of these ions in living organisms over time can lead to serious illnesses.1,3,6,7 Therefore, the development of novel materials and techniques for the highly selective and sensitive detection of heavy metal ions is currently of great importance.1,8–12Since the concept of a fluorescence chemosensor was described by Sousa and Larson,13 many papers involving fluorescent chemosensors for heavy metal ions have been published.14–20 These studies suggest that the use of fluorescent chemosensors for detection of some heavy metal ions can avoid expensive classical methodologies and can allow in situ and real-time detection. Recently, the design and synthesis of innovative materials containing organic functional molecules as fluorescence chemosensors for the detection of heavy metal ions have been of considerable interest with respect to environmental cleanup and the possible recyclability of the organic fluorophores.21–23
Researchers have found that some organic functional molecules can exhibit excellent determination properties for heavy metal ions after being grafted onto silica materials.24–28 The ordered mesoporous SBA-15 is a silica material that possesses many advantages such as a stable mesoporous structure, a tunable pore size, and a high specific surface area with abundant Si–OH active bonds on the pore walls. Some fluorescent chemosensors for heavy metal ions have been reported that are covalently or noncovalently bonded with SBA-15.29,30
In a previous study, we developed an organic/inorganic hybrid material and achieved outstanding response capability.31 The current study seeks to expand the range of applications of the hybrid materials. Accordingly, 5-(4-carboxy-phenylazo)-8-hydroxyquinoline (CPA-8-HQL) was anchored within the channel of mesoporous material SBA-15 for the detection of heavy metal ions. The resulting CPA-8-HQL/SBA-15 hybrid exhibited excellent sensitivity and selectivity to Pb2+ in aqueous media.
Experimental
Materials
8-Hydroxyquinoline and P123 (EO20PO70EO20, Mw = 5800) were purchased from Aldrich. (3-Aminopropyl)triethoxysilane (APTES) was procured from Fluka. 4-Aminobenzoic acid, NaNO2, HCl, NaOH, sodium acetate, n-butanol and tetraethyl orthosilicate (TEOS) were procured from Beijing Chemical Reagent Co. (China).Preparation of CPA-8-HQL
4-Aminobenzoic acid (0.617 g, 4.50 mmol) was dissolved in water (4 mL) containing HCl (37%, 0.9 mL), and the temperature of the solution was lowered to 0–5 °C. NaNO2 (0.310 g 4.5 mmol) was dissolved in water (2 mL) and then added dropwise to the above solution. The resulting solution was stirred for 30 min at 0–5 °C to complete diazotization. 8-Hydroxyquinoline (0.65 g, 4.50 mmol) in a 0.5 M aqueous NaOH solution (10 mL) was added dropwise over 40 min, and stirring continued overnight at room temperature. Afterwards, the pH was adjusted to 7.5–8.0 using a dilute aqueous sodium acetate solution. The precipitated dye was collected by filtration. The crude product was recrystallized twice in n-butanol and then dried in a vacuum oven (yield 35%). It was further characterized by 1H NMR, 13C NMR, and elemental analyses. 1H NMR (500 MHz, DMSO-d6): δ ppm 9.33 (d, 1H), 9.01 (m, 1H), 8.14 (d, 2H), 8.05 (t, 3H), 7.78 (q, 1H), 7.23 (d, 1H), (Fig. S1†) 13C NMR (100 MHz, DMSO-d6): δ ppm 167.36, 160.25, 155.03, 149.61, 138.78, 132.29, 131.09, 128.57, 124.07, 122.51, 116.43, 113.18, (Fig. S2†). Anal. calcd for C16H11N3O3 (293.28): C, 65.53; H, 3.78; N, 14.33; found: C, 65.60; H, 3.75; N, 14.41.Preparation and aminopropyl grafting of SBA-15
The mesoporous molecular sieve SBA-15 was prepared according to the literature by using triblock copolymer P123 as the template.32 The aminopropyl grafting of SBA-15 was carried out using APTES as the silylation reagent. Approximately 0.5 g of SBA-15 powder was mixed with 25 mL of 0.2 mol L−1 APTES chloroform solution and then stirred for 12 h at room temperature. The precipitate was filtered and washed adequately with chloroform. The solid product was air-dried and designated as APTES/SBA-15. According to the results of thermogravimetric analysis, approximately 1.94 mmol g−1 of aminopropyl had been anchored to the inner wall of APTES/SBA-15.Synthesis of the CPA-8-HQL/SBA-15 hybrid system
Forty milligrams of CPA-8-HQL was dissolved in 40 mL of ethanol, and then 0.5 g of APTES/SBA-15 was mixed with the above solution for 30 h at room temperature. The suspension was centrifuged, and the transparent solution was decanted. To remove CPA-8-HQL molecules from the external surface of APTES/SBA-15, the residue was carefully washed with anhydrous ethanol until the filtrate was colorless and there were no characteristic bands of CPA-8-HQL in the UV-vis absorption spectrum. After the suspension was air-dried, a solid red powder was obtained and designated as CPA-8-HQL/SBA-15. On the basis of the results of thermogravimetric analysis, approximately 0.52 mmol g−1 of CPA-8-HQL had been anchored to the inner wall of CPA-8-HQL/SBA-15.Instrumentation
The fluorescence spectra were obtained using a Perkin-Elmer LS 55 spectrofluorophotometer. The UV-vis diffuse reflectance spectra were taken on an Agilent Cary 100 UV-vis recording spectrophotometer with BaSO4 as the reference. The FT-IR transmission spectra were obtained using a Perkin-Elmer Spectrum 100 FT-IR spectrometer equipped with a DTGS detector for KBr pellets. Elemental analyses were performed using a Perkin-Elmer PE 2400 elementar. Transmission electron microscopy (TEM) was carried out using a FEI Tecnai G2 S-Twin transmission electron microscope with a field emission gun operating at 200 kV. X-ray powder diffraction (XRD) spectra were collected on a Siemens D5005 diffractometer with Cu Kα radiation (λ = 1.5418 Å). The samples were step-scanned in 0.02° 2θ steps with a counting time of 2 s per step. 1H NMR (TMS) results were recorded using a Bruker UltraShield 500 MHZ spectrometer. 13C NMR spectra were recorded at 100 MHz. Thermogravimetric analysis (TGA) was carried out using a Perkin-Elmer STA 6000 instrument from ambient temperature to 800 °C with a ramp rate of 10 °C min−1. The N2 adsorption–desorption isotherms were measured using a Micrometrics ASAP 2020 instrument.Results and discussion
To introduce CPA-8-HQL into the channel of mesoporous SBA-15, CPA-8-HQL was mixed with SBA-15 in ethanol for 24 h so that the CPA-8-HQL was physically encapsulated within the channel of SBA-15. However, in subsequent sensing experiments, the CPA-8-HQL detached from the channel of SBA-15. To solve this problem, the inner surface of SBA-15 was first modified with a silylation agent, APTES, and then the CPA-8-HQL molecules were anchored to this pre-modified SBA-15 through an intermolecular hydrogen bond, as shown in Scheme 1.Structural characterization of SBA-15 hybrid
Fig. 1 illustrates the low-angle XRD patterns of SBA-15, APTES/SBA-15, and CPA-8-HQL/SBA-15. All of the samples exhibit three characteristic diffraction peaks that can be indexed to (100), (110), and (200) diffraction associated with typical two-dimensional hexagonal symmetry (P6mm). The results suggest that the functionalization of organic molecules should not affect the original structure of SBA-15. However, the intensities of these characteristic diffraction peaks decrease slightly after the grafting of APTES and decrease further after the anchoring of CPA-8-HQL compared with the unmodified SBA-15. This may be attributed to a reduction in the X-ray scattering contrast between the silica walls and the pore-filling material.33 | ||
| Fig. 1 XRD patterns of SBA-15 (a), APTES/SBA-15 (b) and CPA-8-HQL/SBA-15 (c). Inset shows the amplified part of XRD patterns from 1–3 degrees. | ||
The TEM images shown in Fig. 2a–f compare the pore channel structures of SBA-15, APTES/SBA-15, and CPA-8-HQL/SBA-15, respectively. The TEM images of SBA-15 (Fig. 2a and b) exhibit the highly ordered hexagonal arrays of mesoporous channels. After the grafting of organic molecules, similar hexagonal arrays of ordered channels and the typical honeycomb patterns are clearly visible (Fig. 2c and d for the APTES/SBA-15 and Fig. 2e and f for the CPA-8-HQL/SBA-15). These results indicate that the channel structure of SBA-15 is substantially conserved.
 | ||
| Fig. 2 TEM images of SBA-15 (a and b), APTES/SBA-15 (c and d), and CPA-8-HQL/SBA-15 (e and f) (left side parallel to the pore axis, right side perpendicular to the pore axis). | ||
The changes in porosity and surface physical properties of APTES/SBA-15 and CPA-8-HQL/SBA-15 were further investigated using the N2 adsorption–desorption technique. The adsorption and desorption isotherms of materials (Fig. S3†) all display type IV isotherms with H1-type hysteresis loops at high relative pressure according to the IUPAC classification. This is a characteristic of capillary condensation within uniform pores. A sharp inflection in the P/P0 range from 0.6 to 0.8 is found on isotherms of SBA-15, APTES/SBA-15 and CPA-8-HQL/SBA-15, further evidence that the mesoporous structure was maintained after grafting.34 Table S1† shows the physicochemical properties of SBA-15, APTES/SBA-15 and CPA-8-HQL/SBA-15 as obtained from N2 adsorption–desorption measurements. For mesoporous SBA-15 silica, the BET surface area of 439 m2 g−1, the average pore size of 7.4 nm and the pore volume of 0.66 cm3 g−1 decrease to 378 m2 g−1, 6.5 nm and 0.47 cm3 g−1, respectively, after the samples were grafted with APTES and further decrease to 258 m2 g−1, 6.2 nm and 0.45 cm3 g−1 after functionalization with CPA-8-HQL. The decrease in the BET surface area, pore size and pore volume provide additional evidence of the grafting of the organic molecules to the surface of the inner channel. Because the hysteresis loops are still present in APTES/SBA-15 and CPA-8-HQL/SBA-15 (Fig. S3†), it can be concluded that pore blocking did not happen during the modification process and that the pores are thus still open and accessible for further applications of produced materials.26
The inner assembly details of CPA-8-HQL/SBA-15 have been explored by FT-IR spectroscopy. Fig. 3 depicts the FT-IR spectra of unmodified SBA-15, APTES/SBA-15, CPA-8-HQL/SBA-15 and pure CPA-8-HQL. The typical vibration modes of SBA-15 (ref. 35 and 36) (OH, 3334 cm−1; Si–O–Si, 1088 and 802 cm−1; Si–OH, 953 cm−1; and Si–O, 459 cm−1) are present in all cases (Fig. 3A). However, when compared with the unmodified SBA-15, the relative intensity of the Si–OH vibration at 953 cm−1 decreases in APTES/SBA-15 and CPA-8-HQL/SBA-15, which indicates that after modification, most of the Si–OH bonds on the inner surface of SBA-15 have been occupied. In addition, after the modification, a new band at 1536 cm−1, which is assigned to the N–H bending vibration, appears in APTES/SBA-15 and CPA-8-HQL/SBA-15 (Fig. 3B). In Fig. 3A, a number of sharp vibration peaks appear in the FT-IR spectra of pure CPA-8-HQL, whereas they do not appear in CPA-8-HQL/SBA-15, which suggests that CPA-8-HQL was actually encapsulated within the pores of the host material but not physically adsorbed outside.35
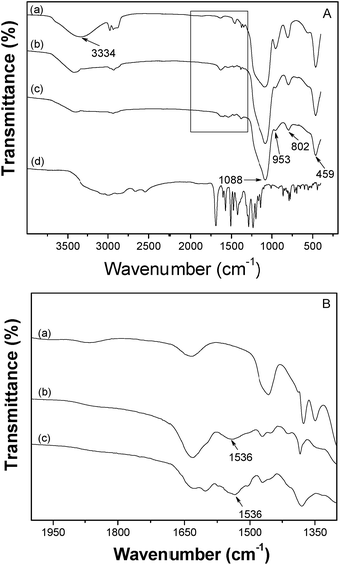 | ||
| Fig. 3 (A): FT-IR spectra of SBA-15 (a), APTES/SBA-15 (b), CPA-8-HQL/SBA-15 (c), and CPA-8-HQL (d). (B): Amplified FT-IR spectra of (a), (b) and (c) curves in the range of 2000 to 1300 cm−1. | ||
To better describe the insertion of CPA-8-HQL into the SBA-15 channel, the UV-vis diffuse-reflectance spectra of CPA-8-HQL and CPA-8-HQL/SBA-15 powder samples were compared (Fig. 4). It can be seen that the characteristic bands of CPA-8-HQL emerge in the spectra of both CPA-8-HQL (Fig. 4a) and CPA-8-HQL/SBA-15 (Fig. 4b): at 210 nm (π → π* electronic transition), at approximately 390 nm (n → π* electronic transition of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –N
N– and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups) and at approximately 500 nm (n → π* electronic transition of carbonyl group). It must be noted that the band at approximately 500 nm has high intensity in the UV-vis diffuse-reflectance spectrum of CPA-8-HQL (Fig. 4a), but it has low intensity after the CPA-8-HQL molecule is encapsulated within the APTES/SBA-15 (Fig. 4b). In the CPA-8-HQL molecule, nonbonded lone-pair electrons in the carbonyl group will undergo a transition from an n orbital to a π* orbital and thus result in the absorption at approximately 500 nm. After CPA-8-HQL is anchored into APTES/SBA-15 through hydrogen bonding, the strong guest–host interaction results in a partial charge transfer (lone pair electron) from the carbonyl group of CPA-8-HQL to the proton of APTES/SBA-15, which decreases the n → π* electronic transition.29 Accordingly, we suggest that CPA-8-HQL was grafted onto the inner surface of SBA-15, as illustrated in Scheme 1.
N– groups) and at approximately 500 nm (n → π* electronic transition of carbonyl group). It must be noted that the band at approximately 500 nm has high intensity in the UV-vis diffuse-reflectance spectrum of CPA-8-HQL (Fig. 4a), but it has low intensity after the CPA-8-HQL molecule is encapsulated within the APTES/SBA-15 (Fig. 4b). In the CPA-8-HQL molecule, nonbonded lone-pair electrons in the carbonyl group will undergo a transition from an n orbital to a π* orbital and thus result in the absorption at approximately 500 nm. After CPA-8-HQL is anchored into APTES/SBA-15 through hydrogen bonding, the strong guest–host interaction results in a partial charge transfer (lone pair electron) from the carbonyl group of CPA-8-HQL to the proton of APTES/SBA-15, which decreases the n → π* electronic transition.29 Accordingly, we suggest that CPA-8-HQL was grafted onto the inner surface of SBA-15, as illustrated in Scheme 1.
Thermogravimetric analysis (TGA) was carried out to calculate the approximate loaded amount of organic moieties on the surface of SBA-15 (Fig. 5). The weight losses at a temperature of approximately 100 °C correspond to the desorption of physisorbed water. Then, the major weight losses of 16.72% and 29.55% between 200 °C and 600 °C are due to the decomposition of organic groups in APTES/SBA-15 and CPA-8-HQL/SBA-15, respectively. Finally, minor weight losses above 600 °C correspond to the dehydroxylation of the Si–OH group. Therefore, the approximate amount of the aminopropyl moiety present in APTES/SBA-15 is calculated to be 1.94 mmol g−1 and the CPA-8-HQL moiety in CPA-8-HQL/SBA-15 is calculated to be 0.52 mmol g−1. Actually, as the reaction between APTES/SBA-15 and CPA-8-HQL is not complete, there are still unreacted aminopropyl groups in CPA-8-HQL/SBA-15.
Effect of pH
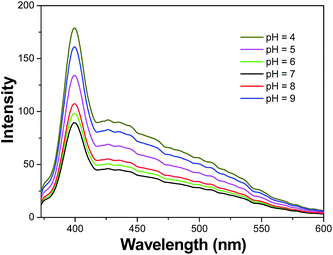
The effect of pH in the range of 4 to 9 on the fluorescence emission of CPA-8-HQL/SBA-15 was tested. As shown in Fig. 6, in a neutral solution (pH = 7.0), CPA-8-HQL/SBA-15 displayed a weak fluorescence emission because the lone pairs of the sp2 nitrogen cause a photoinduced electron transfer (PET) to the fluorophore. In acidic solutions (pH = 4.0, 5.0 and 6.0), the fluorescence intensity of CPA-8-HQL/SBA-15 increased, most likely due to the inhibition of PET from the lone pairs of sp2 nitrogen to the fluorophore. However, in alkaline solutions (pH = 8.0 and 9.0), the fluorescence of CPA-8-HQL/SBA-15 also increased. We believe this is due to deprotonation of the phenolic hydroxyl group, which otherwise quenches the fluorescence by vibrational coupling of the excited state to water.37 Therefore, the pH was adjusted to 7 using HEPES buffer (10 mM) in subsequent experiments. In addition, working at pH 7.0 can ensure that the nitrogen remains unprotonated and can participate in the binding of metal ions if required.Sensing properties
To evaluate CPA-8-HQL/SBA-15 as a selective fluorescence sensor for a particular metal ion, the influences of different metal ions (Li+, Na+, K+, Ca2+, Mg2+, Ba2+, Mn2+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Cd2+, Al3+ and Ag+) on the relative changes in the fluorescence spectra of the CPA-8-HQL/SBA-15 aqueous suspension were studied. As shown in Fig. 7, the addition of Pb2+ led to an obvious enhancement of the fluorescence intensity of CPA-8-HQL/SBA-15 at 398 nm. The coordination of Pb2+ with CPA-8-HQL occupies the lone pairs of nitrogen; thus, the PET from nitrogen to the fluorophore is inhibited and a high fluorescence emission can be obtained.It is noteworthy that the addition of Pb2+ also induces a distinct new emission band at a longer wavelength (λmax = 429 nm), indicating a prominent π–π stacking after coordination.38 The coordination of CPA-8-HQL with Pb2+ draws fluorophores closer together resulting in the enhancement of π–π stacking, and therefore, the fluorescence intensities at longer wavelengths increase. The particularly high fluorescence at 429 nm resulting from the presence of Pb2+ provides the hybrid of CPA-8-HQL/SBA-15 with the ability to detect Pb2+ selectively. An obvious blue-purple emission can be observed visually for the aqueous solution of CPA-8-HQL/SBA-15 upon addition of Pb2+ when compared with that of only CPA-8-HQL/SBA-15 (inset in Fig. 7).
Sensitivity and detection limit
To assess the ability of CPA-8-HQL/SBA-15 to sense metal ions, the changes in the fluorescence intensity were measured at 429 nm after addition of different metal ions. The results are shown in Fig. 8. The value of ΔI/I0 is used as the sensitivity parameter, which is the value of metal ion-induced change in fluorescence at 429 nm normalized with respect to the blank sample (CPA-8-HQL/SBA-15 included no any metal ion). As shown in Fig. 8, the largest ΔI/I0 (approximately 2.32) is observed in the presence of Pb2+, while there were small changes observed in the presence of other metal ions. Therefore, CPA-8-HQL/SBA-15 displays a high sensitivity for Pb2+.To evaluate the sensing behavior of CPA-8-HQL/SBA-15, its fluorescence in an aqueous suspension as a function of the Pb2+ concentration was measured (Fig. 9). Continuous titration of CPA-8-HQL/SBA-15 with Pb2+ induces a dramatic increase in the fluorescence spectra such as those shown in Fig. 9. The detection limit39,40 was calculated from the titration data to be 4.90 × 10−7 mol L−1 for Pb2+ (Fig. S4†).
Selectivity
When evaluating the performance of a fluorescence chemosensor, high selectivity toward the analytes over the other competitive species coexisting in the sample is an extremely important feature.28,41 Accordingly, the influence of a number of common cations on the sensing ability of CPA-8-HQL/SBA-15 was investigated by treating the CPA-8-HQL/SBA-15 aqueous solution with 10 equiv. of Pb2+ in the presence of 10 equiv. of background metal ions and then measuring the corresponding fluorescence spectrum. The results, which are shown in Fig. 10, confirm that background metal ions show little or no obvious interference with the detection of Pb2+. This fact suggests that CPA-8-HQL/SBA-15 could recognize Pb2+ with high selectivity against other metal ions.Preliminary analytical application
The practical applicability of the proposed sensing system was evaluated by the detection of Pb2+ in drinking water, tap water and river water samples. First, the water samples were filtered to remove insoluble substances. Then, Pb2+ was added to the samples, and the pH of each sample was adjusted to 7 using HEPES buffer (10 mM). Finally, the Pb2+ content of each sample was measured. The observed results (given in Table 1) show that CPA-8-HQL/SBA-15 is able to measure the concentrations of Pb2+ with good recovery.| Sample | Pb2+ added (μmol L−1) | Pb2+ found Pb2+ (μmol L−1) | Recovery (%) |
|---|---|---|---|
| a Mean value of three determinations.b Standard deviation. | |||
| Drinking water | |||
| 1 | 0 | — | — |
| 2 | 1 | 0.98 | 98.0a ± 0.2b |
| 3 | 10 | 10.01 | 100.1a ± 0.3b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Tap water | |||
| 1 | 0 | — | — |
| 2 | 1 | 1.01 | 101.0a ± 0.1b |
| 3 | 10 | 9.99 | 99.9a ± 0.2b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| River water | |||
| 1 | 0 | — | — |
| 2 | 1 | 0.97 | 97.0a ± 0.3b |
| 3 | 10 | 10.02 | 100.2a ± 0.1b |
Conclusions
In conclusion, an organic/inorganic hybrid chemosensor based on the SBA-15 mesoporous silica has been developed. A series of characteristic results demonstrate that the organic molecule CPA-8-HQL has been successfully grafted to the channel of SBA-15 and that the mesoporous structure has been perfectly preserved. The sensing ability of CPA-8-HQL/SBA-15 to metal ions was examined by fluorescence spectroscopy, and the results show a high selectivity and sensitivity toward Pb2+ in aqueous media. The organic/inorganic chemosensor may be an important material for future application in the field of heavy metal ion detection because it will facilitate environmental cleanup and the possible recyclability of the organic molecules. Moreover, this strategy is certainly not limited to CPA-8-HQL and SBA-15, but it can be extended to many other chemosensors and other silica materials.Acknowledgements
This work is supported by the China NSFC (51308151), Natural Science Foundation of Heilongjiang Province of China (QC2013C046).Notes and references
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS PubMed.
- C. Orvig and M. J. Abrams, Chem. Rev., 1999, 99, 2201–2203 CrossRef CAS PubMed.
- R. McRae, P. Bagchi, S. Sumalekshmy and C. J. Fahrni, Chem. Rev., 2009, 109, 4780–4827 CrossRef CAS PubMed.
- H. W. Rhee, H. Y. Choi, K. Han and J. I. Hong, J. Am. Chem. Soc., 2007, 129, 4524–4525 CrossRef CAS PubMed.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008–2012 CrossRef CAS PubMed.
- A. P. de Silva, D. B. Fox, A. J. M. Huxley and T. S. Moody, Coord. Chem. Rev., 2000, 205, 41–57 CrossRef CAS.
- M. Wennberg, T. Lundh, I. A. Bergdahl, G. Hallmans, J. H. Jansson, B. Stegmayr, H. M. Custodio and S. Skerfving, Environ. Res., 2006, 100, 330–338 CrossRef CAS PubMed.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS.
- S. K. Kim, S. H. Lee, J. Y. Lee, J. Y. Lee, R. A. Bartsch and J. S. Kim, J. Am. Chem. Soc., 2004, 126, 16499–16506 CrossRef CAS PubMed.
- J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780–3799 CrossRef CAS PubMed.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- L. R. Sousa and J. M. Larson, J. Am. Chem. Soc., 1977, 99, 307–310 CrossRef CAS.
- D. Guo, Z. P. Dong, C. Luo, W. Y. Zan, S. Q. Yan and X. J. Yao, RSC Adv., 2014, 4, 5718–5725 RSC.
- H. R. Xu, K. Li, Q. Liu, T. M. Wu, M. Q. Wang, J. T. Hou, Z. Huang, Y. M. Xie and X. Q. Yu, Analyst, 2013, 138, 2329–2334 RSC.
- H. B. Li and H. J. Yan, J. Phys. Chem. C, 2009, 113, 7526–7530 CAS.
- Y. Mikata, A. Takekoshi, A. Kizu, Y. Nodomi, M. Aoyama, K. Yasuda, S. Tamotsu, H. Konno and S. C. Burdette, RSC Adv., 2014, 4, 12849–12856 RSC.
- Y. L. Hung, T. M. Hsiung, Y. Y. Chen, Y. F. Huang and C. C. Huang, J. Phys. Chem. C, 2010, 114, 16329–16334 CAS.
- H. Li, S. J. Zhang, C. L. Gong, Y. F. Li, Y. Liang, Z. G. Qi and S. Chen, Analyst, 2013, 138, 7090–7093 RSC.
- K. Tiwari, M. Mishra and V. P. Singh, RSC Adv., 2013, 3, 12124–12132 RSC.
- C. X. Song, X. L. Zhang, C. Y. Jia, P. Zhou, X. Quan and C. Y. Duan, Talanta, 2010, 81, 643–649 CrossRef CAS PubMed.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403–1419 CrossRef CAS.
- A. P. Wight and M. E. Davis, Chem. Rev., 2002, 102, 3589–3614 CrossRef CAS PubMed.
- S. Areti, D. S. Yarramala, K. Samanta, V. K. Hinge, J. Khedkar and C. P. Rao, RSC Adv., 2014, 4, 16290–16297 RSC.
- J. Isaad and A. E. Achari, Tetrahedron, 2013, 69, 4866–4874 CrossRef CAS PubMed.
- P. Zarabadi-Poor, A. Badiei, A. A. Yousefi and J. Barroso-Flores, J. Phys. Chem. C, 2013, 117, 9281–9289 CAS.
- X. J. Wan, S. Yao, H. Y. Liu and Y. W. Yao, J. Mater. Chem. A, 2013, 1, 10505–10512 CAS.
- Z. P. Dong, X. Tian, Y. Z. Chen, J. R. Hou and J. T. Ma, RSC Adv., 2013, 3, 2227–2233 RSC.
- L. Gao, Y. Wang, J. Q. Wang, L. Huang, L. Y. Shi, X. X. Fan, Z. G. Zou, T. Yu, M. Zhu and Z. S. Li, Inorg. Chem., 2006, 45, 6844–6850 CrossRef CAS PubMed.
- J. Q. Wang, L. Huang, M. Xue, Y. Wang, L. Gao, J. H. Zhu and Z. G. Zou, J. Phys. Chem. C, 2008, 112, 5014–5022 CAS.
- L. Y. Zhao, S. C. Wang, Y. Wu, Q. F. Hou, Y. Wang and S. M. Jiang, J. Phys. Chem. C, 2007, 111, 18387–18391 CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- S. Gago, J. A. Fernandes, J. P. Rainho, R. A. S. Ferreira, M. Pillinger, A. A. Valente, T. M. Santos, L. D. Carlos, P. J. A. Ribeiro-Claro and I. S. Gonçalves, Chem. Mater., 2005, 17, 5077–5084 CrossRef CAS.
- F. Zhang, Y. Yan, H. Yang, Y. Meng, C. Yu, B. Tu and D. Zhao, J. Phys. Chem. B, 2005, 109, 8723–8732 CrossRef CAS PubMed.
- S. Li, H. W. Song, W. L. Li, X. G. Ren, S. Z. Lu, G. H. Pan, L. B. Fan, H. Q. Yu, H. Zhang, R. F. Qin, Q. L. Dai and T. Wang, J. Phys. Chem. B, 2006, 110, 23164–23169 CrossRef CAS PubMed.
- P. P. Yang, S. S. Huang, D. Y. Kong, J. Lin and H. G. Fu, Inorg. Chem., 2007, 46, 3203–3211 CrossRef CAS PubMed.
- J. F. Callan, A. P. de Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551–8588 CrossRef CAS PubMed.
- F. Galindo, M. Becerril, I. Burgette, S. V. Luis and L. Viagra, Tetrahedron Lett., 2004, 45, 1659–1662 CrossRef CAS PubMed.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 68, 1414–1418 CrossRef CAS.
- A. Caballero, R. Martínez, V. Lloveras, I. Ratera, J. Vidal-Gancedo, K. Wurst, A. Tárraga, P. Molina and J. Veciana, J. Am. Chem. Soc., 2005, 127, 15666–15667 CrossRef CAS PubMed.
- F. Y. Yan, D. L. Cao, N. Yang, Q. H. Yu, M. Wang and L. Chen, Sens. Actuators, B, 2012, 162, 313–320 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR of CPA-8-HQL, 13H NMR spectra of CPA-8-HQL, N2 adsorption–desorption experiments and details of calculation procedure for detection limit. See DOI: 10.1039/c5ra00696a |
| This journal is © The Royal Society of Chemistry 2015 |