DOI:
10.1039/C5RA00617A
(Paper)
RSC Adv., 2015,
5, 17872-17878
Four-fold concentration of sucrose in sugarcane juice through energy efficient forward osmosis using sea bittern as draw solution†
Received
12th January 2015
, Accepted 4th February 2015
First published on 5th February 2015
Abstract
Clarified sugarcane juice (osmotic coefficient, φ, ∼1.01) was efficiently dewatered through the spontaneous process of Forward Osmosis (FO) employing sea bittern as draw solution. Sea bittern is the mother liquor that remains after recovery of common salt from seawater. It is either discarded to sea or evaporated to higher densities in solar salt pans for recovery of other marine chemicals such as bromine, Epsom salt, potash and magnesium chloride. Compared to seawater (φ = 0.905) which has limited potential as draw solution, the φ values of the sea bittern samples were in the range of 1.41 to 3.24, providing thereby a high osmotic drive. A polyamide thin film composite membrane was used in the study. With 1 bar applied pressure, room temperature operation, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 volume ratio of sugar cane juice to bittern (φ = 2.26; concentrations of main constituents (% w/v): Na+ = 2.83, K+ = 2.03, Mg2+ = 7.42, Cl− = 23.48, SO42− = 8.42), sucrose concentration in the juice was enhanced from 10.5% (w/v) to 40.6% (w/v) over 4 h, with average flux of 13 L m−2 h−1. Sucrose loss was <3%. Energy computations indicated a saving of 69 kg of bagasse per m3 of raw juice, assuming all process energy (steam/electricity) is derived from bagasse. Epsom salt of high purity was recovered from the spent draw solution upon chilling.
8 volume ratio of sugar cane juice to bittern (φ = 2.26; concentrations of main constituents (% w/v): Na+ = 2.83, K+ = 2.03, Mg2+ = 7.42, Cl− = 23.48, SO42− = 8.42), sucrose concentration in the juice was enhanced from 10.5% (w/v) to 40.6% (w/v) over 4 h, with average flux of 13 L m−2 h−1. Sucrose loss was <3%. Energy computations indicated a saving of 69 kg of bagasse per m3 of raw juice, assuming all process energy (steam/electricity) is derived from bagasse. Epsom salt of high purity was recovered from the spent draw solution upon chilling.
1. Introduction
Although the classical, energetically favoured phenomenon of osmosis predates reverse osmosis, the latter has gained increasingly in importance. Osmosis has, however, begun receiving growing attention in recent years, albeit by the new name of forward osmosis (FO). Interest in FO emanates largely from the need to conserve energy in process technologies.1 Focus areas of interest in FO are mainly seawater desalination, waste water reclamation and protein enrichment. Energy generation through FO is also being investigated. Besides research on design of suitable membranes with high rejection efficiency and permeate flux, much effort has been devoted to the identification of suitable draw solutions having high osmotic coefficient. Ge et al. have reviewed recently the different types of draw solutions used by various researchers and technologists.2 Selection of solutes to be used in draw solution hinges on the specific application area and the strategy to be adopted. Broadly, the solutes fall into the categories of volatile compounds (e.g., ammonium bicarbonate),3 nutrient compounds,4 inorganic salts,5 and organic salts.6 Use of hydrophilic magnetic nanoparticles is also reported.7,8 For high volume, low value applications, suitable draw solutions will be required that are abundant and cost-effective. The draw solution must also be benign and possess high osmotic coefficient to drive the desired extent of concentration of a feed. The quest for ideal draw solutions is viewed in a recent review as the “Holy Grail” of forward osmosis.9
Sucrose (sugar) is a high volume commodity. Much of it is produced from sugarcane. Raw juice expelled from the cane contains 9–12% (w/v) sucrose.10 It is stabilized to prevent decay, clarified to remove suspended solids, and then subjected to multiple effect evaporation to obtain sugar syrup containing 50–60% sucrose by weight. Further concentration under vacuum yields a supersaturated solution, from which sucrose is crystallized upon seeding. Thermal energy for the process is largely met from bagasse. However, if the energy requirement can be reduced, then more bagasse would be available for other purposes such as paper manufacturing, power generation and animal feed. Sucrose present in by-product molasses is utilised in substantial quantity for the production of bio-ethanol.11,12 Thus the energy output to input ratio of bioethanol too would be impacted by energy conservation in the process of sugar and molasses recovery from sugarcane juice. Concentration of the juice in energy efficient manner is therefore a desirable goal. The most common means resorted to increase sugar concentration in the juice is the “burn and crop” method adopted in the field itself. This method is gradually being phased out as it is not considered to be eco-friendly.13 Other approaches include reverse osmosis at high applied pressure (40 bar) and membrane distillation.14,15 Although in comparison to multiple-effect evaporation, there may be some energy savings, these methods too are energy intensive.16 Studies have been reported on concentration of pure sugar solutions through the energy efficient process of FO, using NaCl as draw solution.17 However, to our knowledge, there are no reports on concentration of sugarcane juice by FO.
The present study demonstrates such concentration in practical manner employing an indigenously developed thin film composite polyamide membrane and abundant sea bittern as draw solution. Sea bittern refers to the multi-electrolyte mother liquor that remains after common salt production from seawater, ca. 1 m3 of virgin bittern being generated per ton of salt. It is either discarded to sea or evaporated to higher densities in solar salt pans for recovery of other marine chemicals such as bromine, Epsom salt, potash and magnesium chloride, generating more concentrated bitterns from these processes. There are no reports, however, on the utility of bittern as a source of latent energy to drive FO.
2. Experimental section
2.1. Materials
Standard sucrose, glucose and fructose were purchased from SISCO Laboratories Pvt Ltd, Mumbai, India and used without further purification. Activated charcoal was procured from SRL, Mumbai. Raw sugarcane juice, without additives, was obtained from a local juice parlor. The raw juice was clarified by treating with 2% activated charcoal followed by filtering. If required, the clarified juice was treated with lime water to adjust the pH to ca. 8. NaCl used for preparation of brackish water feed for RO experiments with the prepared membrane was obtained from RFCL, New Delhi, India. The bittern samples used were as follows: B1, sea bittern of 28 °Bé density [°Bé = 145(1 − 1/ρ), where ρ = specific gravity] obtained from Tata Chemicals Limited, Mithapur, India; B2, sea bittern of 32.5–33.5 °Bé obtained through concentration of B1; B3, sea bittern of 36.3 °Bé obtained from the Institute's experimental salt farm after kainite (KCl·MgSO4·3H2O) mixed salt crystallization; B4, end bittern of 38.9 °Bé obtained from B3.
2.2. Membrane fabrication
A high flux thin-film composite (TFC) polyamide membrane developed indigenously was used in all the FO experiments of the present study. Membrane was synthesised following an earlier report.18 Non-woven polyester fabric (80–85 g m−2) was procured from AWA, Japan and polysulfone layer was cast from a solution containing 14.5% polysulfone (Solvay Advanced Polymers, India) in DMF, using a semi-automatic casting machine. The polyamide coating over polysulfone was prepared in a semi-automatic coating machine through controlled interfacial polymerization of m-phenylenediamine (3% w/v in H2O) and trimesoyl chloride (0.125% in hexane).
2.3. Forward osmosis
A laboratory scale flat sheet membrane testing kit with 0.0057 m2 active membrane surface area was fabricated in the Institute's workshop. Pressure booster pumps capable of maintaining a pressure between 0–10 bars were used for circulation of feed solution and draw solution taken in suitably sized glass containers. After several trials, an optimized inlet operating pressure of 1 bar was adopted. A restricting needle valve was provided on the feed outlet of the membrane kit to pressurize the feed solution. For the circulation of both feed solution and draw solution, RO booster pumps (KEMFLO) were used with nominal flow rate of 1.8 LPM and maximum input pressure capacity of 60 psi. Membrane active layer (AL) facing both feed solution and draw solution was similar throughout all experiments (5.3 cm × 10.7 cm) and cross flow velocity was calculated to be 0.283 m s−1. A picture of the unit is shown in Fig. 1.
 |
| | Fig. 1 Photograph of FO assembly employed in the present study. | |
2.4. Recovery of Epsom salt (MgSO4·7H2O) of higher purity from B2 after dilution during FO
In a typical experimental procedure 2 L of B2 was taken as draw solution and 250 mL of freshly prepared clarified sugarcane juice (10.5% (w/v) sucrose) was taken as feed solution. The FO process was terminated when the volume of the feed solution was reduced to 50–60 mL, and the draw solution volume rose simultaneously by ca. 10% (v/v). This diluted B2 was chilled at 5 ± 2 °C to crystallize Epsom salt. Epsom salt recovery from undiluted draw solution was taken as control to ascertain relative purities. Yield was expressed with respect to sulphate content in B2 after FO, this being the limiting reactant.
2.5. Methods
Concentrations of Mg2+ and Ca2+ were estimated by EDTA titration, K+ and Na+ by flame photometry (Cole-Parmer Instrument Company Chicago, Model 2655-00, Digital flame Analyzer), and SO42− and Cl− by ion chromatography (Dionex 500) or classical techniques. Field emission scanning electron microscopy (FESEM LEO 1430VP) was used to examine the surface morphology of the membrane. FTIR spectra of the samples were recorded with a Perkin-Elmer spectrum GX series 59387 FT-IR spectrophotometer. Total dissolved solids (TDS) in feed and permeate obtained from RO experiments were measured using conductivity meter (CON700, EUTECH Instruments). Back diffusion of draw solution constituents into feed solution was studied using an inductively coupled plasma optical emission spectrometer (ICP-OES, Perkin-Elmer, Optima 2000). Concentrations of sugars were determined using high performance liquid chromatography (HPLC, Waters (Alliance) and Shimadzu), employing Aminex-HPX-87P (BioRad, USA) column. Milli Q water was used as eluant. The flow rate was 0.6 mL min−1 and temperature was 85 °C. Sugar samples for HPLC were prepared by dilution with Milli Q water and 50 μL of the solution was injected into the column.
2.6. Water activity, permeate flux and % concentration of feed
Water activity plays a crucial role in inducing high osmotic gradient of a multi-electrolyte mixture. It can be defined as ratio of the vapour pressure (P) in solution to the vapour pressure of pure water (P0) [eqn (1)].| | |
aw = P/P0 = ERH (%)/100
| (1) |
The solvent flux (Jn) across the FO membrane was calculated using eqn (2).
| |
 | (2) |
In this equation,
Jn is the flux (L m
−2 h
−1),
Vt0 and
Vtn are the volumes (L) of feed solution at zero time and at time interval
n, respectively, and
A is area (m
2) of the membrane.
Rejection efficiency in RO experiments was calculated using eqn (3).
| |
 | (3) |
R is the rejection efficiency,
Cf the concentration of feed at time
t and
C0 the initial feed concentration.
2.7. Energy computation
Energy computation for concentration of sugarcane juice by forward osmosis was carried out assuming industrial model. Concentration of 1 m3 sugarcane juice to 0.25 m3 through FO is feasible with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 volume ratio of juice to draw solution (B2). Further, if an average permeate flux of 13 L m−2 h−1 is considered as demonstrated in the present study, the task can be accomplished in 1 h if an FO unit having active membrane area of 57.7 m2 is employed. The requirement of pressure at feed side was 1 bar whereas the draw side was at atmospheric pressure. Densities of sugarcane juice before and after FO were 1.12 and 1.52 g cm−3, respectively. Initial and final viscosities were in the range of 3–6 cP, and initial and final flow rates were 10 m3 h−1 and 7.5 m3 h−1, respectively. Thus, to generate 1 bar pressure total developed water column required is in the range of 11.2 to 15.2 m. A 2 HP LEAKLESS LCP-30 centrifugal pump can generate 10–25 m water column and 5–12 m3 h−1 flow rate and the same is considered for the purpose of computation of energy required for pumping of the feed.19 Now, energy consumption for this pump = (2 × 0.746 × 1) kW h = 1.492 kW h or 5.37 MJ (1 HP = 0.746 kW; 1 kW h = 3.6 MJ). Since, initially 1 m3 sugarcane juice would be pumped, so energy requirement per m3 of sugarcane juice processed = 5.37/7.5 MJ = 0.72 MJ. In the draw side, the initial and final volume before and after FO did not change significantly, thus effect of density and viscosity was assumed unchanged. Since the volume ratio of draw solution to feed solution is taken as 8
8 volume ratio of juice to draw solution (B2). Further, if an average permeate flux of 13 L m−2 h−1 is considered as demonstrated in the present study, the task can be accomplished in 1 h if an FO unit having active membrane area of 57.7 m2 is employed. The requirement of pressure at feed side was 1 bar whereas the draw side was at atmospheric pressure. Densities of sugarcane juice before and after FO were 1.12 and 1.52 g cm−3, respectively. Initial and final viscosities were in the range of 3–6 cP, and initial and final flow rates were 10 m3 h−1 and 7.5 m3 h−1, respectively. Thus, to generate 1 bar pressure total developed water column required is in the range of 11.2 to 15.2 m. A 2 HP LEAKLESS LCP-30 centrifugal pump can generate 10–25 m water column and 5–12 m3 h−1 flow rate and the same is considered for the purpose of computation of energy required for pumping of the feed.19 Now, energy consumption for this pump = (2 × 0.746 × 1) kW h = 1.492 kW h or 5.37 MJ (1 HP = 0.746 kW; 1 kW h = 3.6 MJ). Since, initially 1 m3 sugarcane juice would be pumped, so energy requirement per m3 of sugarcane juice processed = 5.37/7.5 MJ = 0.72 MJ. In the draw side, the initial and final volume before and after FO did not change significantly, thus effect of density and viscosity was assumed unchanged. Since the volume ratio of draw solution to feed solution is taken as 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a LEAKLESS LCP-70 pump with 65 m3 h−1 flow rate and 20 HP power is required for pumping of draw solution. Now, energy consumption for this pump = (20 × 0.746 × 1) kW h = 14.92 kW h or 53.7 MJ. Since 8–9 m3 draw solution would be pumped per m3 of sugarcane juice processed by FO, so energy requirement = (53.7/65) × 8 MJ = 6.61 MJ. Thus total power requirement in the process is 7.33 MJ. Assuming 20% efficiency of biomass gasification during power generation,20 and considering a calorific value of 19 MJ kg−1 of bagasse, ca. 2 kg of bagasse is required to generate the required power.
1, a LEAKLESS LCP-70 pump with 65 m3 h−1 flow rate and 20 HP power is required for pumping of draw solution. Now, energy consumption for this pump = (20 × 0.746 × 1) kW h = 14.92 kW h or 53.7 MJ. Since 8–9 m3 draw solution would be pumped per m3 of sugarcane juice processed by FO, so energy requirement = (53.7/65) × 8 MJ = 6.61 MJ. Thus total power requirement in the process is 7.33 MJ. Assuming 20% efficiency of biomass gasification during power generation,20 and considering a calorific value of 19 MJ kg−1 of bagasse, ca. 2 kg of bagasse is required to generate the required power.
The energy requirement to concentrate 1 m3 sugarcane juice to 0.25 m3 under industrial model using 3 stages multiple effect evaporation (MEE) was also calculated for comparison with FO. Assuming 80% efficiency in 3 stage-MEE,21 0.512 kg steam is required to evaporate 1 kg of water. Energy requirement for evaporation of 0.75 m3 water from 1 m3 sugarcane juice = (750 × 0.512 × 540) kcal = 207![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 kcal or 867 MJ. Assuming 65% thermal efficiency of steam generation using bagasse,20,22 requirement of the biomass is ca. 71 kg.
360 kcal or 867 MJ. Assuming 65% thermal efficiency of steam generation using bagasse,20,22 requirement of the biomass is ca. 71 kg.
3. Results and discussions
3.1. Membrane characterization
The Fig. 2 provides information on the morphology and FT-IR spectrum of the chosen membrane. ATR-FTIR pattern in Fig. 2(c) confirms the polyamide layer formation on polyester–polysulfone support. The characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands at 1660 cm−1 (amide-I stretch) is seen whereas the peak at around 1550 cm−1 corresponds to N–C
O bands at 1660 cm−1 (amide-I stretch) is seen whereas the peak at around 1550 cm−1 corresponds to N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N–H bending (amide-II). The peak at 786 cm−1 in TFC is assigned to amide V.
O and C–N–H bending (amide-II). The peak at 786 cm−1 in TFC is assigned to amide V.
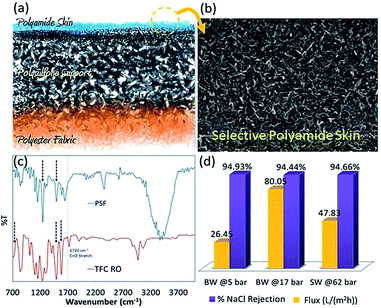 |
| | Fig. 2 (a) Schematic depiction of TFC membrane prepared as per the experimental procedure described in the Experimental section; (b) SEM surface image of polyamide layer; (c) FTIR spectra of polysulfone (PSF) support layer and TFC membrane; (d) bar diagrams depicting salt rejection and flux data of the RO membrane for tests conducted with BW and SW at different applied pressures. | |
Initially, TFC membranes were characterized for their separation efficacy under RO conditions. When operated in RO mode at 5 bar pressure, a flux of 26.45 L m−2 h−1 and rejection of 94.9% were observed for desalination of 2000 mg L−1 (ppm) brackish water (BW). High rejection efficiency was observed for desalination of seawater (SW) also, albeit at high applied pressure. In view of the satisfactory flux and rejection even at low (5 bar) operating pressure in experiments with BW, the membrane was considered suitable for FO application and results obtained with the membrane are discussed below. The performance of the TFC membrane was tested in FO mode using 1 M NaCl as draw solution and pure water as feed solution. At 1 bar applied pressure the pure water flux was found to be 2.4 L m−2 h−1 with negligible crossover of solute (49 ppm) from draw solution to feed solution.
3.2. Sea bittern as draw solution
Table 1 provides detailed compositions of the four seawater bittern samples employed in the present study. Data on ionic strength (I), osmotic coefficient (φ) and viscosity (η) are also provided. Except in the initial sample, wherein the principal constituent was NaCl, other bittern samples comprised MgCl2 as the main constituent. Fig. 3 shows the plot of water activity (aw) of seawater and the four bittern samples. As can be seen from the figure, starting from the value of 0.87 of aw for seawater, the value reduced to 0.72 for the virgin bittern (B1) obtained after recovery of common salt. Thereafter, aw dropped steeply for B2 to B4. The osmotic coefficient (φ) is related to aw through eqn (4).23 In this equation, ϑi is the number of ions arising from a given salt, and mi is the molality of each of the salts in the multi-electrolyte system. The values of φ of the four bittern samples are plotted in Fig. 3. B1 gave φ = 1.41 and the value increased all the way up to 3.24 for B4, the latter sample containing nearly 50% (w/v) MgCl2. There are prior reports on the use of pure MgCl2 in FO. For example, Loeb et al. had conducted a study on FO with commercial asymmetric cellulose acetate membranes supported on fabric, wherein 6% and 12% (w/v) aqueous MgCl2 was used as draw solution with deionized water as feed, respectively.24 The flux was found to be 0.42 L m−2 h−1, the low value being attributed to the resistance posed by the fabric. In another study 0.36 M MgCl2 was utilised as draw solution, with deionized water as feed. The flux was estimated to be 8.4 L m−2 h−1. To effect an efficient FO process, osmotic coefficient of the draw solution is more critical than the purity of solute used. Thus whereas a 21% aqueous solution of pure MgCl2 gave a pure water flux of 4.4 L m−2 h−1 when employed as draw solution, the corresponding flux was 5.5 L m−2 h−1 with B2 bittern which contained similar concentration of MgCl2, and other salts additionally. No study is, however, reported on use of bittern as draw solution in FO, even though it should be highly efficient for this purpose by virtue of its high φ value, and further considering its abundance, affordability and benign nature.| |
 | (4) |
Table 1 Chemical composition, ionic strength (I) and viscosity (η) of seawater bittern samples used as draw solutions in the present study
| Sample |
°Bé/(ρ) |
Na+ |
K+ |
Mg2+ |
Ca2+ |
Cl− |
SO42− |
I |
φ |
η |
| (% w/v) |
M |
cp |
| Below detection limit. |
| B1 |
28.0/(1.240) |
6.83 |
1.31 |
3.63 |
0.02 |
27.48 |
4.51 |
9.46 |
1.41 |
3.1 |
| B2 |
33.5/(1.300) |
2.83 |
2.03 |
7.42 |
BDLa |
23.48 |
8.42 |
12.04 |
2.26 |
5.4 |
| B3 |
36.3/(1.334) |
0.44 |
0.34 |
10.74 |
0.14 |
30.02 |
0.43 |
13.36 |
2.98 |
7.2 |
| B4 |
38.9/(1.367) |
0.29 |
0.09 |
13.74 |
0.17 |
38.88 |
3.74 |
17.72 |
3.24 |
13.1 |
 |
| | Fig. 3 Plot of water activity and osmotic coefficient of different seawater bitterns vs. °Bé/ρ. | |
3.3. Studies on FO with pure sugar solutions
Fig. 1 shows a photograph of the experimental assembly employed in FO experiments. The membrane area was 0.0057 m2. The experiments were conducted over 5 h at room temperature in the cell described above, with recirculation of feed solution and draw solution. Studies were initially carried out with 6% (w/v) sucrose solution at 0.5, 1 and 1.5 bar pressure. 1 bar was found to be optimum. Fig. 4 shows a bar diagram of the average flux at 1 bar pressure obtained for FO employing 1 L of 6% sucrose (φ = 1.006)25 as feed solution and 0.2 L of B1–B4 as draw solution. Expectedly, B1 gave the lowest average flux of 7 ± 2 L m−2 h−1 whereas the value was nearly double for B4. The other bittern samples gave intermediate values. However, considering the low ratio of draw solution to feed solution in the experiment of Fig. 4, all the bittern samples can be considered to have good potential for dewatering. HPLC analysis of the draw solution was undertaken after FO to ascertain sucrose transport across the membrane. As can be seen from the data in Fig. S1,† passage of sucrose from feed solution to draw solution was negligible. Although sucrose is the main constituent in sugarcane juice, glucose and fructose are also reported to be present in small amounts. Accordingly, a comparative FO study of 6% sucrose, 6% glucose (φ = 1.007) and 6% (φ = 1.007)25 fructose was undertaken employing B4 as draw solution (Fig. S2†). Average fluxes and final concentrations of sugars measured after 5 h of FO run indicated highest flux for sucrose. This is likely on account of the fact that sucrose is a disaccharide whereas glucose and fructose are monosaccharides.
 |
| | Fig. 4 Average flux (over 5 h) for FO of 6% sucrose feed using different bittern samples as draw solution. The experiments were conducted at room temperature and 1 bar applied pressure at feed solution side. | |
3.4. Concentration of sugarcane juice
The studies were extended to FO of sugarcane juice. The juice used in the study contained 9.7% (w/v) sucrose, 0.32% (w/v) glucose and 0.43% (w/v) fructose and FO experiments were conducted with raw and clarified juices. B4 was used as draw solution in the initial studies and experiments were conducted at 1 bar pressure with 1 L feed solution and 0.2 L draw solution at room temperature over 5 h, with constant circulation of feed solution and draw solution. As can be seen from Fig. 5(a), the permeate flux was poor when raw juice was used.
 |
| | Fig. 5 (a) Permeate water flux versus time for room temperature FO experiments conducted with raw-, UF-processed and charcoal-treated sugarcane juice (9.7% w/v initial sucrose) as feed solution (1 L) and B4 as draw solution (0.2 L); (b) average flux and final concentration of sucrose in feed solution after FO over 5 h; (c) HPLC profiles of charcoal-treated sugarcane juice prior to commencement of FO, sugarcane juice after FO and B4 after FO, where (1) sucrose, (2) glucose, and (3) fructose. | |
When the juice was clarified through ultrafiltration or charcoal treatment, the flux improved markedly and remained fairly steady after 3 h. Fig. 5(b) provides data of average flux and final sucrose concentration after completion of the experiments. Fig. 5(c) shows that the profiles of sucrose, glucose and fructose remained virtually the same before and after FO, albeit with enhancement in the absolute sucrose concentration from 9.7% to 19.3% (φ = 1.03),25 i.e., ca. two-fold concentration. HPLC of the draw solution after FO is shown in Fig. 5(c). The loss of sucrose in the draw solution was estimated to be <3%.
3.5. Inorganic solute permeation
Back diffusion of salts from draw solution to feed solution may decrease the net osmotic pressure across the membrane, with adverse consequence on performance with progress of FO. Build-up of salts in the sugarcane juice may also adversely affect its processing.26 Accordingly, an assessment was made of Na+, K+, Ca2+, Mg2+, Cl−, and SO42− in the feed solution after FO for the experiments reported in Sections 3.3 and 3.4 using B4 bittern as draw solution. As can be seen from Table 2, the concentrations of all of the above ions in the pure sugar solutions after FO were minimal, notwithstanding their presence in B4 (Table 1). The anion and cation charge balance was found to be in good agreement in all the cases. Thus the membrane prepared for the study showed excellent resistance to sugar transport in forward direction and inorganic ion transport in reverse direction, while allowing facile permeation of water. Mineral compositional analyses were also generated for concentrated sugarcane juice after the FO experiment in Section 3.4, and the results compared with that of the pristine juice (Table 2). The data are provided in Table 2. In this case too, back diffusion was only marginal.
Table 2 Concentrations of mineral constituents in feed solutions after ca. two-fold concentration by FO over 5 h using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of draw solution to feed solutiona
5 ratio of draw solution to feed solutiona
| Feed solution |
Draw solution |
Back diffusion of ions to feed solution after 5 h of FO run |
| SO42− (% w/v) |
Cl− (% w/v) |
Na+ (% w/v) |
K+ (% w/v) |
Ca2+ (% w/v) |
Mg2+ (% w/v) |
| Figures in parentheses correspond to equivalents of charge per litre based on the concentration data. |
| Glucose (6%) |
B4 |
0.048 (0.01) |
0.18 (0.051) |
0.051 (0.022) |
0.0012 (0.0003) |
0.038 (0.019) |
0.035 (0.029) |
| Fructose (6%) |
B4 |
0.05 (0.01) |
0.24 (0.068) |
0.056 (0.024) |
0.0033 (0.0008) |
0.045 (0.023) |
0.049 (0.041) |
| Sucrose (6%) |
B4 |
0.045 (0.009) |
0.17 (0.048) |
0.062 (0.027) |
0.0051 (0.0013) |
0.032 (0.016) |
0.023 (0.019) |
| Clarified sugarcane juice |
B4 |
0.14 (0.029) |
0.36 (0.101) |
0.103 (0.045) |
0.01 (0.0026) |
0.077 (0.039) |
0.036 (0.03) |
| Pristine sugarcane juice |
0.068 (0.014) |
0.11 (0.031) |
0.0064 (0.003) |
0.016 (0.004) |
0.061 (0.031) |
0.017 (0.014) |
3.6. Value addition of draw solution with four-fold concentration of sugarcane juice
Although B4 exhibited the highest osmotic coefficient (Fig. 3) and efficiency as draw solution, the flux was moderately good with the other bittern samples also (Fig. 4). These too, therefore, merit consideration if they offer any specific advantage. B2 contains high concentrations of both Mg2+ and SO42−, and can be utilised for the selective recovery of Epsom salt (an important commodity chemical) by chilling. It is reported that the purity of Epsom salt is much improved when the bittern is diluted to the extent of ca. 10% with fresh water.27,28 Such dilution would be achieved without additional effort or expenses in the course of FO. Moreover, it is more practical in solar salt works to concentrate bittern up to the stage of B2 than B4.27 Thus an experiment was proposed with B2 bittern. While designing the experiment, a target of four-fold concentration of sugarcane juice was set. Considering 10–12% initial sucrose, this would mean a final concentration of 40–48%, which is close to the concentration (50–60%) achieved through multiple effect evaporation in commercial operation prior to the final evaporation step for sucrose crystallization. Considering initial volume of feed solution = (V1)F, initial sucrose concentration in feed solution = (C1)F, final volume of feed solution = (V2)F, final sucrose concentration in feed solution = (C2)F, initial volume of draw solution = (V1)D and final volume of draw solution = (V2)D, the following relationships (eqn 5(a)–(g)) enabled computation of (V1)D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (V1)F to satisfy both objectives simultaneously. Sucrose loss was not considered.
(V1)F to satisfy both objectives simultaneously. Sucrose loss was not considered.| | |
(C2)F = 4 × (C1)F (considering 4-fold concentration)
| (5a) |
| | |
(V1)F × (C1)F = (V2)F × (C2)F = (V2)F × 4 × (C1)F
| (5b) |
| | |
Hence, (V2)F = 0.25 × (V1)F
| (5c) |
| | |
Permeate volume during FO = (V1)F − 0.25(V1)F = 0.75(V1)F
| (5d) |
| | |
(V2)D = (V1)D + 0.75(V1)F = 1.1 × (V1)D (considering 10% dilution of draw solution)
| (5e) |
| | |
Hence, 0.1(V1)D = 0.75(V1)F
| (5f) |
| | |
i.e., (V1)D/(V1)F = 7.5
| (5g) |
An experiment was carried out accordingly with 250 mL of charcoal-treated sugarcane juice (10.5% w/v initial sucrose content) as feed solution and 2 L of B2 as draw solution. After 4 h of FO with continuous recirculation, the sucrose concentration in dewatered juice was found to increase to 40.6% (φ = 1.052),25 i.e., by a factor of ca. 4, with average flux of 8.2 L m−2 h−1. Sucrose crossover into the draw solution was confirmed to be negligible (Fig. S3†). Epsom salt was recovered from the draw solution after FO in 54% average yield (sulphate basis) with >98% purity. The purity of the Epsom salt from B2 as such was 83–84%.
3.7. Energy saving
A comparative assessment was made of the energy requirement for four-fold concentration of sugarcane juice through the processes of multiple effect evaporation and FO process as in Section 3.6. The details are provided in the Experimental section. Assuming commercial scale operation, the requirement of electrical energy to concentrate 1 m3 sugarcane juice to 0.25 m3 by FO is only 7.33 MJ, (which is equivalent to 37 MJ of calorific value) whereas the same extent of concentration through multiple effect evaporation (MEE) requires 867 MJ of thermal energy.
In most sugarcane industries the thermal/electrical energy requirement is met by combustion/gasification of bagasse.29 Considering a calorific value of 19 MJ per kg of bagasse, and 65% thermal efficiency, 71 kg of bagasse must be sacrificed for 4-fold concentration of 1 m3 of juice through MEE. Thus the net saving in bagasse – which can be put to alternative uses – would be 69 kg per m3 of sugarcane juice processed, i.e., a saving of ca. 97.2%. A previous report on RO-based concentration has indicated a saving of ca. 86% through a multi-stage RO process at 32 bar pressure and 80 °C temperature.30 However, no detailed methodology was presented. Besides the high expenditure on capital cost, the above reported figure of energy saving is lower than the saving achieved in the present study. Moreover, it is likely that the problem of membrane fouling would be more severe at higher applied pressure.
4. Conclusions
Efficient concentration of sugarcane juice through forward osmosis was demonstrated in the present study. The process utilised abundant sea bittern as a novel high osmotic coefficient draw solution that is benign and cost-effective at the same time. A high flux thin film composite polyamide membrane, operating at a nominal pressure of 1 bar, was utilised for this purpose. Using an appropriate ratio of draw solution to sugarcane juice, up to 4-fold concentration of sucrose in the latter was achieved at room temperature in re-circulation mode, with <3% sucrose loss. Back diffusion of solutes from the draw solution to feed solution was shown to be negligible. 40% of the bagasse obtained from sugarcane can be conserved by switching from forced evaporation to the proposed route. Another novel feature was that the bittern sample utilised as draw solution in the finalized scheme benefited from its dilution during forward osmosis. Such dilution raised the purity of Epsom salt recovered upon chilling. Thus the application reported herein constitutes one of the few examples where FO has an added value. The process of the present study merits consideration in locations where sugarcane is grown in reasonable proximity to salt works.
Acknowledgements
The referees are acknowledged for their valuable comments. SKN acknowledges the Department of Science and Technology, Government of India for the DST-INSPIRE Faculty Award and Research Grant (IFA12-CH-84). DM and KKG acknowledge CSIR and UGC, respectively, for NET Fellowship. Authors thank the Analytical Discipline for all round instrumentation facility. Mr Jagat Kalotra and Mr Jayanti bhai Rathod are acknowledged for their technical assistance. This is CSIR-CSMCRI communication #107/2014.
Notes and references
- Z. Liu, H. Bai, J. Lee and D. D. Sun, Energy Environ. Sci., 2011, 4, 2582–2585 Search PubMed.
- Q. Ge, M. Ling and T.-S. Chung, J. Membr. Sci., 2013, 442, 225–237 CrossRef PubMed.
- T. Y. Achilli and A. E. Cath, J. Membr. Sci., 2010, 364, 233–241 CrossRef PubMed.
- S. Phuntsho, H. K. Shon, S. K. Hong, S. Y. Lee and S. Vigneswaran, J. Membr. Sci., 2011, 375, 172–181 CrossRef PubMed.
- P. McCormick, J. Pellegrino, F. Mantovani and G. Sarti, J. Membr. Sci., 2008, 325, 467–478 CrossRef PubMed.
- Q. Ge, J. C. Su, G. Amy and T. S. Chung, Water Res., 2012, 46, 1318–1326 CrossRef PubMed.
- M. M. Ling, K. Y. Wang and T. S. Chung, Ind. Eng. Chem. Res., 2010, 49, 5869–5876 CrossRef.
- M. M. Ling, T. S. Chung and X. M. Lu, Chem. Commun., 2011, 47, 10788–10790 RSC.
- B. V. der Bruggen and P. Luis, Rev. Chem. Eng., 2014 DOI:10.1515/revce-2014-0033.
- H. Zabed, G. Faruq, J. N. Sahu, M. S. Azirun, R. Hashim and A. N. Boyce, Bioethanol Production from Fermentable Sugar Juice, Sci. World J., 2014, vol. 2014, 957102 Search PubMed.
- O. S. Marina, T. Dias, L. Junqueira, C. Eduardo, V. Rossell, R. M. Filho and A. Bonomi, Fuel Process. Technol., 2013, 109, 84–89 CrossRef PubMed.
- G. P. da Silva, E. F. de Araújo, D. O. Silva and W. V. Guimarães, Braz. J. Microbiol., 2005, 36, 395–404 CrossRef PubMed.
- J. E. D. Cançado, P. H. N. Saldiva, L. A. A. Pereira, L. B. L. S. Lara, P. Artaxo, L. A. Martinelli, M. A. Arbex, A. Zanobetti and A. L. F. Braga, Environ. Health Perspect., 2006, 114, 725–729 CrossRef.
- S. Nene, S. Kaur, K. Sumod, B. Joshi and K. S. M. S. Raghavarao, Desalination, 2002, 147, 157–160 CrossRef.
- A. Hinkova, Z. Bubník, P. Kadlec and J. Pridal, Sep. Purif. Technol., 2002, 26, 101–110 CrossRef.
- S. Lee, C. Boo, M. Elimelech and S. Hong, J. Membr. Sci., 2010, 365, 34–39 CrossRef PubMed.
- M. E. G. Castello, J. R. McCutcheon and M. Elimelech, J. Membr. Sci., 2009, 338, 61–66 CrossRef PubMed.
- P. S. Singh, S. V. Joshi, J. J. Trivedi, C. V. Devmurari, A. P. Rao and P. K. Ghosh, J. Membr. Sci., 2006, 278, 19–25 CrossRef PubMed.
- S. Chantasiriwan, TIJSAT, 2013, 18, 10–21 Search PubMed.
- Energy and Environmental Analysis, Inc., an ICF International Company, and Eastern Research Group, Inc. (ERG) for the U. S. Environmental Protection Agency, Combined Heat and Power Partnership, September 2007, EPA Combined Heat and Power Partnership, Biomass CHP Catalog, pp 60.
- J. G. Brennan, Evaporation and Dehydration in Food Processing Handbook, ed. J. G. Brennan and A. S. Grandison, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2006, ch. 3, pp. 71–121 Search PubMed.
- Energy and Environmental Analysis, Inc., an ICF International Company, and Eastern Research Group, Inc. (ERG) for the U. S. Environmental Protection Agency, Combined Heat and Power Partnership, September 2007, EPA Combined Heat and Power Partnership, Biomass CHP Catalog, pp 60. EPA Combined Heat and Power Partnership, Biomass CHP Catalog, pp. 41.
- G. Lychnos, J. P. Fletcher and P. A. Davies, Desalination, 2010, 250, 172–178 CrossRef PubMed.
- S. Loeb, L. Titleman, E. Korngold and J. Frieman, J. Membr. Sci., 1997, 129, 243–249 CrossRef.
- Z.-N. Gao, J.-F. Li and X.-L. Wen, Indian J. Chem., 2002, 41A, 1184–1187 Search PubMed.
- S. K. Thampy, P. K. Narayanan, G. S. Trivedi, D. K. Gohil and V. K. Indushekhar, Int. Sugar J., 1999, 101(1207), 365–367 Search PubMed.
- K. K. Ghara, N. Korat, D. Bhalodia, J. Solanki, P. Maiti and P. K. Ghosh, RSC Adv., 2014, 4, 34706–34711 RSC.
- J. A. Fernandez-Lozano, Northern Ohio Geological Society, 1974, 2, 501–510 Search PubMed.
- J. Goldemberg, Biotechnol. Biofuels, 2008, 1, 6–13 CrossRef PubMed.
- S. Gul and M. Harasek, Energy Savings in Sugar Manufacturing with the Implementation of a new Membrane Process, http://www.nt.ntnu.no/users/skoge/prost/proceedings/pres2011-and-icheap10/PRES11/231Gul.pdf, 2009.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00617a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 volume ratio of sugar cane juice to bittern (φ = 2.26; concentrations of main constituents (% w/v): Na+ = 2.83, K+ = 2.03, Mg2+ = 7.42, Cl− = 23.48, SO42− = 8.42), sucrose concentration in the juice was enhanced from 10.5% (w/v) to 40.6% (w/v) over 4 h, with average flux of 13 L m−2 h−1. Sucrose loss was <3%. Energy computations indicated a saving of 69 kg of bagasse per m3 of raw juice, assuming all process energy (steam/electricity) is derived from bagasse. Epsom salt of high purity was recovered from the spent draw solution upon chilling.
8 volume ratio of sugar cane juice to bittern (φ = 2.26; concentrations of main constituents (% w/v): Na+ = 2.83, K+ = 2.03, Mg2+ = 7.42, Cl− = 23.48, SO42− = 8.42), sucrose concentration in the juice was enhanced from 10.5% (w/v) to 40.6% (w/v) over 4 h, with average flux of 13 L m−2 h−1. Sucrose loss was <3%. Energy computations indicated a saving of 69 kg of bagasse per m3 of raw juice, assuming all process energy (steam/electricity) is derived from bagasse. Epsom salt of high purity was recovered from the spent draw solution upon chilling.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 volume ratio of juice to draw solution (B2). Further, if an average permeate flux of 13 L m−2 h−1 is considered as demonstrated in the present study, the task can be accomplished in 1 h if an FO unit having active membrane area of 57.7 m2 is employed. The requirement of pressure at feed side was 1 bar whereas the draw side was at atmospheric pressure. Densities of sugarcane juice before and after FO were 1.12 and 1.52 g cm−3, respectively. Initial and final viscosities were in the range of 3–6 cP, and initial and final flow rates were 10 m3 h−1 and 7.5 m3 h−1, respectively. Thus, to generate 1 bar pressure total developed water column required is in the range of 11.2 to 15.2 m. A 2 HP LEAKLESS LCP-30 centrifugal pump can generate 10–25 m water column and 5–12 m3 h−1 flow rate and the same is considered for the purpose of computation of energy required for pumping of the feed.19 Now, energy consumption for this pump = (2 × 0.746 × 1) kW h = 1.492 kW h or 5.37 MJ (1 HP = 0.746 kW; 1 kW h = 3.6 MJ). Since, initially 1 m3 sugarcane juice would be pumped, so energy requirement per m3 of sugarcane juice processed = 5.37/7.5 MJ = 0.72 MJ. In the draw side, the initial and final volume before and after FO did not change significantly, thus effect of density and viscosity was assumed unchanged. Since the volume ratio of draw solution to feed solution is taken as 8
8 volume ratio of juice to draw solution (B2). Further, if an average permeate flux of 13 L m−2 h−1 is considered as demonstrated in the present study, the task can be accomplished in 1 h if an FO unit having active membrane area of 57.7 m2 is employed. The requirement of pressure at feed side was 1 bar whereas the draw side was at atmospheric pressure. Densities of sugarcane juice before and after FO were 1.12 and 1.52 g cm−3, respectively. Initial and final viscosities were in the range of 3–6 cP, and initial and final flow rates were 10 m3 h−1 and 7.5 m3 h−1, respectively. Thus, to generate 1 bar pressure total developed water column required is in the range of 11.2 to 15.2 m. A 2 HP LEAKLESS LCP-30 centrifugal pump can generate 10–25 m water column and 5–12 m3 h−1 flow rate and the same is considered for the purpose of computation of energy required for pumping of the feed.19 Now, energy consumption for this pump = (2 × 0.746 × 1) kW h = 1.492 kW h or 5.37 MJ (1 HP = 0.746 kW; 1 kW h = 3.6 MJ). Since, initially 1 m3 sugarcane juice would be pumped, so energy requirement per m3 of sugarcane juice processed = 5.37/7.5 MJ = 0.72 MJ. In the draw side, the initial and final volume before and after FO did not change significantly, thus effect of density and viscosity was assumed unchanged. Since the volume ratio of draw solution to feed solution is taken as 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a LEAKLESS LCP-70 pump with 65 m3 h−1 flow rate and 20 HP power is required for pumping of draw solution. Now, energy consumption for this pump = (20 × 0.746 × 1) kW h = 14.92 kW h or 53.7 MJ. Since 8–9 m3 draw solution would be pumped per m3 of sugarcane juice processed by FO, so energy requirement = (53.7/65) × 8 MJ = 6.61 MJ. Thus total power requirement in the process is 7.33 MJ. Assuming 20% efficiency of biomass gasification during power generation,20 and considering a calorific value of 19 MJ kg−1 of bagasse, ca. 2 kg of bagasse is required to generate the required power.
1, a LEAKLESS LCP-70 pump with 65 m3 h−1 flow rate and 20 HP power is required for pumping of draw solution. Now, energy consumption for this pump = (20 × 0.746 × 1) kW h = 14.92 kW h or 53.7 MJ. Since 8–9 m3 draw solution would be pumped per m3 of sugarcane juice processed by FO, so energy requirement = (53.7/65) × 8 MJ = 6.61 MJ. Thus total power requirement in the process is 7.33 MJ. Assuming 20% efficiency of biomass gasification during power generation,20 and considering a calorific value of 19 MJ kg−1 of bagasse, ca. 2 kg of bagasse is required to generate the required power.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 kcal or 867 MJ. Assuming 65% thermal efficiency of steam generation using bagasse,20,22 requirement of the biomass is ca. 71 kg.
360 kcal or 867 MJ. Assuming 65% thermal efficiency of steam generation using bagasse,20,22 requirement of the biomass is ca. 71 kg.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands at 1660 cm−1 (amide-I stretch) is seen whereas the peak at around 1550 cm−1 corresponds to N–C
O bands at 1660 cm−1 (amide-I stretch) is seen whereas the peak at around 1550 cm−1 corresponds to N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N–H bending (amide-II). The peak at 786 cm−1 in TFC is assigned to amide V.
O and C–N–H bending (amide-II). The peak at 786 cm−1 in TFC is assigned to amide V.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of draw solution to feed solutiona
5 ratio of draw solution to feed solutiona
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (V1)F to satisfy both objectives simultaneously. Sucrose loss was not considered.
(V1)F to satisfy both objectives simultaneously. Sucrose loss was not considered.




