Complexation of triblock reverse copolymer 10R5 with surface active ionic liquids in aqueous medium: a physico-chemical study†
Abstract
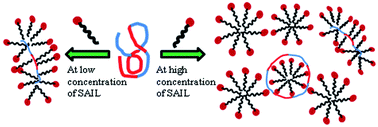
A comprehensive study on the interactions of surface active ionic liquids (SAILs) 1-alkyl-3-methyl imidazolium chlorides, [Cnmim][Cl], where n = 8, 10, and 12, with a triblock reverse copolymer, 10R5, [(PPO)8–(PEO)22–(PPO)8] has been performed using various physico-chemical techniques viz. surface tension, conductivity, isothermal titration calorimetry (ITC), turbidity, fluorescence spectroscopy, and dynamic light scattering (DLS). The interactions between triblock reverse copolymer with SAILs have been emphasized in terms of three concentration regions due to different modes of interactions between them whereas the previous studies reported that interactions between cationic surfactants and triblock copolymers are moderately weak. Different transitions corresponding to different stages of interactions of 10R5 with SAILs are observed from different techniques. Various thermodynamic parameters are calculated using conductivity and ITC measurements, whereas fluorescence studies have provided useful information about the polarity of the cybotactic region of the probe in the complexes formed by 10R5 and SAILs. The size of polymer–SAIL complexes has been investigated using dynamic light scattering and ITC measurements. The results obtained from different techniques have been correlated with each other to get a concise picture regarding the type of interactions prevailing between polymer and SAILs.

 Please wait while we load your content...
Please wait while we load your content...