Thermally enhanced self-propelled droplet motion on gradient surfaces†
Abstract
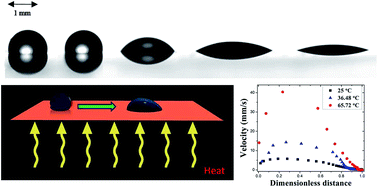
Significant enhancements in the instantaneous speed of a water droplet on a silicon surface with a chemically induced hydrophilicity gradient are observed with moderate increases in substrate temperature. The instantaneous droplet velocities and the contact angles are measured by a frame by frame analysis of the droplet motion using a goniometer with a high speed camera. A force balance based model captures the underlying experimental trends in a precise quantitative sense. The relevant forces are the chemically induced surface tension gradient inspired driving force and the resistive forces namely, the three-phase contact line, hydrodynamic and the drag force. The variation in the values of the coefficient of contact line friction and its effect on the overall droplet transport has been evaluated. This study points to the enhanced cooling potential of speciality surfaces where the dissipated heat may be utilized as a natural advantage for faster movement of droplets towards the hot spot.

 Please wait while we load your content...
Please wait while we load your content...