Open Access Article
Open Access ArticleA continuous method to prepare poorly crystalline silver-doped calcium phosphate ceramics with antibacterial properties
S.
Range
a,
D.
Hagmeyer
a,
O.
Rotan
a,
V.
Sokolova
a,
J.
Verheyen
b,
B.
Siebers
b and
M.
Epple
*a
aInorganic Chemistry and Center for Nanointegration Duisburg-Essen (CeNIDE), University of Duisburg-Essen, Universitaetsstr. 5-7, 45117 Essen, Germany. E-mail: matthias.epple@uni-due.de
bBiofilm Centre Molecular Enzyme Technology and Biochemistry, University of Duisburg-Essen, Universitaetsstr. 5-7, 45117 Essen, Germany
First published on 7th May 2015
Abstract
Silver-doped calcium phosphate ceramics were prepared in discontinuous and continuous processes with different amounts of incorporated silver (up to 1.8 wt% silver). In particular, the effects of pH, reaction time and light exposure on the incorporation of silver into the calcium phosphate ceramic were investigated. In the dark, silver can be incorporated as colourless silver ions (Ag+) into the apatite lattice, but the integration occurs slowly. Under ambient light, a rapid photoreduction to elemental silver (Ag0) occurs which permits a continuous process to prepare silver-doped calcium phosphate ceramics. The silver-doped calcium phosphate ceramics were characterized by scanning electron microscopy, X-ray powder diffraction, infrared spectroscopy, thermogravimetry, and elemental analysis (Ca, Ag, phosphate). The silver release from the silver-doped calcium phosphate ceramics was measured by a combination of dialysis and atomic absorption spectroscopy. The antimicrobial effect was tested on bacteria (Escherichia coli), and the cytotoxic effect was tested on HeLa cells (human epithelial cervical cancer cells). For comparison, stoichiometric silver phosphate, Ag3PO4, was prepared. The release of silver from silver phosphate is much faster, leading to pronounced antibacterial but also cytotoxic effects.
Introduction
Of the most prominent calcium phosphate ceramics, hydroxyapatite (HA) is one of the most frequently used bone substitution materials, due to its similarity to the mineral in mammalian bones.1–5 To combat infections after implantation, silver has been proposed as an antibacterial agent6 and as an additive to calcium phosphate ceramics.7–25 This antibacterial effect has been suggested to combat bacterial colonization (i.e. biofilm formation) on implants. The properties of silver-doped calcium phosphate ceramics that are reported in the literature are diverging with respect to the chemical nature of the incorporated silver (e.g. as a calcium-substituting cation or as nanoscopic metallic silver), and the synthesis conditions are not always fully reported with respect to light exposure and reaction conditions. It was our aim to obtain a full and comprehensive picture of the different ways to prepare silver-doped calcium phosphates, employing a wide range of analytical methods. For this, the precipitation of silver-doped calcium phosphate was carried out both in a batch synthesis and in a continuous synthesis under strict control of the reaction conditions.26Results and discussion
The easiest way to prepare silver-doped calcium phosphate is a batch synthesis, i.e. the precipitation of calcium phosphate in the presence of a soluble silver salt. We have systematically studied this process that depends on time, temperature and light exposure. Aqueous solutions of Ca(NO3)2, (NH4)2HPO4 and AgNO3 were mixed and stirred for different times under various conditions. The solid precipitate was isolated by centrifugation and analysed by X-ray powder diffraction, scanning electron microscopy and visual inspection.If the batch precipitation was carried under complete light exclusion at pH 9, the precipitate remained white, indicating an incorporation of Ag+ ions into the calcium phosphate lattice. If the pH was allowed to drop below 9 during the reaction, an ill-defined yellow powder, consisting of poorly crystalline apatite and silver phosphate was obtained.
The integration of silver into the calcium phosphate ceramics was slow and of limited extent, pointing to a slow exchange of Ca2+ ions by Ag+ ions in the dispersion (Fig. 1). Note that under these conditions, calcium phosphate rapidly precipitates and therefore a silver incorporation can only occur by ion exchange in contact of the solid with the surrounding solution. To balance the charge when one calcium ion Ca2+ is substituted by one silver ion Ag+, it is likely that one phosphate anion PO43− is protonated to a hydrogen phosphate anion HPO42−, leading to a calcium-deficient hydroxyapatite CDHA.27,28
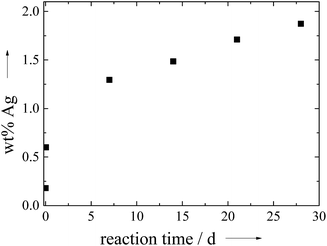 | ||
Fig. 1 Incorporation of silver into calcium phosphate ceramics as a function of the reaction time under complete light exclusion (pH 9, molar ratio (Ag + Ca)/P = 1.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and molar ratio Ag 1 and molar ratio Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 Ca = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). 9). | ||
If the pH was raised above 9 by adding ammonia, no silver incorporation was observed, even at longer times, probably due to the formation of the diammine silver complex [Ag(NH3)2]+.
Under light exposure (daylight, no special illumination), the initially white precipitate rapidly turned grey which is an indication for the formation of metallic silver by photoreduction as ionic silver is colourless. The amount of incorporated silver increased from 0.2 wt% after a few seconds to 3.16 wt% after 3 h. This demonstrates that reduced silver, probably as silver nanoparticles, has a much higher tendency to enter the solid calcium phosphate phase.
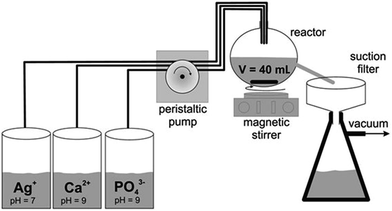
Due to the much faster incorporation of silver under light exposure, we developed a continuous process to obtain silver-doped calcium phosphate with a high content of silver. The experimental setup is shown in Fig. 2.
The solid product was isolated by filtration and dried at 70 °C in air. For comparison, pure silver phosphate, Ag3PO4, was prepared in the same way.
The obtained silver-doped calcium phosphate particles were small and had an irregular shape. In contrast, Ag3PO4 consisted of smooth, μm-sized crystals (Fig. 3).
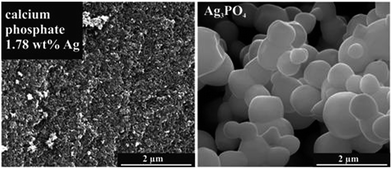 | ||
| Fig. 3 SEM images of silver-doped calcium phosphate with 1.78 wt% silver and of pure silver phosphate. | ||
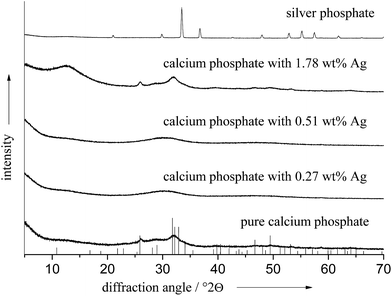
X-ray powder diffraction confirmed these observations. We found a poorly crystalline apatite phase in all cases, sometimes even X-ray amorphous. The crystallinity was not significantly influenced by the presence of silver as the diffractogram of the pure calcium phosphate ceramics shows. No diffraction peaks of elemental silver or silver phosphate were detectable. This indicates that the silver is not present as a distinct phase with sufficient particle size or crystallinity. In contrast, Ag3PO4 showed sharp diffraction peaks, confirming the high crystallinity (Fig. 4).
Table 1 gives all analytical data for the prepared ceramics. The molar ratio of Ca/P in the products was in the range of 1.51 to 1.60, and the molar ratio of (Ca + Ag)/P was of the same order of magnitude. The amount of silver that was incorporated into the calcium phosphate phase was dependent on the silver concentration in solution. Between 1 and 4 mol% of silver in the initial solution, silver was not detected in the calcium phosphate ceramics. Above 4 mol% silver in the solution, silver was incorporated in an almost linear relationship (Fig. 5), up to 1.78 wt% silver in the ceramic. Notably, the silver content in the solid ceramic was always lower than that in the solution.
| Silver content/mol% Ag+ in the solution | Elemental analysis of the dried silver-doped calcium phosphate ceramics | ||||
|---|---|---|---|---|---|
| Ca2+/wt% | PO43−/wt% | Ag+/wt% | Ca/P molar ratio | (Ca + Ag)/P molar ratio | |
| 0 | 33.5 | 54.5 | 0.00 | 1.54 | 1.54 |
| 1 | 33.9 | 51.6 | 0.00 | 1.56 | 1.56 |
| 2 | 34.2 | 52.6 | 0.00 | 1.54 | 1.54 |
| 3 | 34.0 | 52.4 | 0.00 | 1.53 | 1.53 |
| 4 | 33.0 | 50.5 | 0.00 | 1.55 | 1.55 |
| 5 | 34.2 | 50.7 | 0.27 | 1.60 | 1.60 |
| 6 | 34.2 | 51.5 | 0.70 | 1.57 | 1.59 |
| 7 | 32.8 | 49.4 | 0.51 | 1.58 | 1.58 |
| 8 | 33.5 | 51.4 | 0.73 | 1.54 | 1.56 |
| 9 | 33.0 | 49.4 | 0.89 | 1.59 | 1.60 |
| 10 | 32.5 | 51.2 | 1.78 | 1.51 | 1.54 |
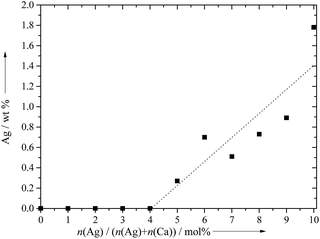 | ||
| Fig. 5 The content of silver in the calcium phosphate ceramics, precipitated under light exposure at pH 9 in a continuous process during 30 min. | ||
We ascribe the fact that silver was not included at low concentrations to the formation of the soluble silver diammine complex [Ag(NH3)2]+. The pH of the calcium and phosphate solutions was adjusted to 9 with aqueous ammonia solution before the experiment. The concentration of ammonia was around 10 mM after the dilution in the precipitation vessel.
The contents of silver and phosphate in Ag3PO4 were 78.6 wt% and 21.4 wt%, respectively, corresponding well to the stoichiometric values (77.3 and 22.7 wt%).
In the IR spectra we observed the expected bands at 3400 cm−1 for H2O, at 1625 cm−1 for the OH stretching vibration, at 1385 cm−1 for the carbonate stretching vibration, and at about 1040 and 560 cm−1 for the phosphate vibrations. These values are consistent with the values given in the literature.1,15,29–31 The IR spectrum of silver phosphate corresponded to the literature.32 The phosphate bands of pure AgPO4 are at 551 cm−1 and 1114 cm−1.
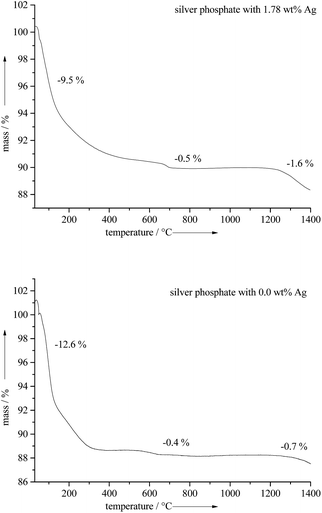
Thermogravimetric analysis of the silver-doped calcium phosphate ceramics gave between 9 to 13 wt% of water (released up to 400 °C) and 0.5 to 0.7 wt% CO2 (released from 500–700 °C). Note that some carbonate was incorporated into the calcium phosphate ceramics due to the precipitation in air (Fig. 6).26
The calcination of the silver-doped calcium phosphate ceramics at 850 °C led to β-tricalcium phosphate (β-TCP) with a comparable silver content. Heating to 1300 °C led to a mixture of α- and β-TCP with almost no silver content. Obviously, silver evaporates under these conditions (Table 2 and Fig. 6).
| Temperature/°C | Ca2+/wt% | PO43−/wt% | Ag+/wt% |
|---|---|---|---|
| 850 | 38.53 | 57.70 | 1.71 |
| 1300 | 39.13 | 61.20 | 0.20 |
This indicates that it is not possible to increase the sintering temperature of such ceramics to very high temperature, e.g. to obtain a silver-doped biphasic calcium phosphate because silver evaporates (Tfus = 961.8 °C).
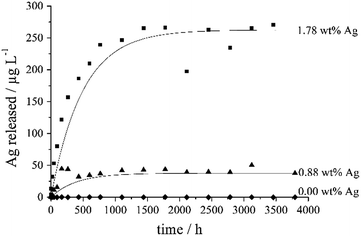
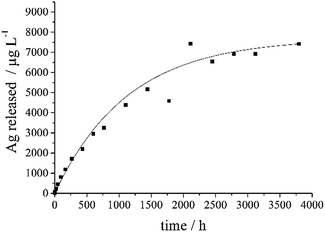
Silver ions are the biologically active component with respect to bacteria and cells.6 Therefore, a silver-doped calcium phosphate exerts its bactericidal effect through the release of silver ions. The release of silver from the silver-doped calcium phosphate ceramics samples was studied by dialysis according to ref. 33 and 34. The silver concentration remained constant after about 1000 h (Fig. 7).
The pellet with 1.78 wt% Ag contained about 3.5 mg Ag, of which about 130 μg (3.7%) were released over time. The pellet with 0.88 wt% Ag contained about 1.8 mg Ag, of which about 20 μg (1.1%) were released over time. As the nature of the incorporated silver (ionic substitution or metallic nanoparticle) is not quantitatively known, the release kinetics probably indicate a combination of a dissolution (ionic silver) and an oxidation (metallic silver). The pellet of Ag3PO4 contained about 157 mg Ag, of which about 2 mg (1.3%) were released over time (Fig. 8). Note that silver phosphate is a sparingly water-soluble inorganic salt.35
In Fig. 9, the effect of silver-doped ceramics, Ag3PO4 and silver-free calcium phosphate on the Gram-negative bacterium Escherichia coli is shown. In order to reduce the dilution effect of the liquid volume, agar diffusion assays instead of liquid cultures were used, which represent established models for bacterial unsaturated biofilms at the solid/air interface and allow a qualitative analysis. The formation of inhibition zones indicates the antibacterial effect. The antibacterial effects of the pellets of silver-doped calcium phosphate and of silver phosphate were clearly visible. As expected, the pure calcium phosphate pellet showed no inhibition zone and thus no anti-bacterial effect.
The cytotoxic concentrations of ionic silver are 0.5–5 mg L−1 for bacteria (minimum inhibitory concentration) and 1.5–2.5 mg L−1 for eukaryotic cells.6,36 Except for Ag3PO4, these values were not reached in the dialysis experiments, but clearly, the effective silver concentration depends on the volume of the surrounding liquid. In this regard, the dialysis experiment with 500 mL was probably far from the conditions in the agar test.
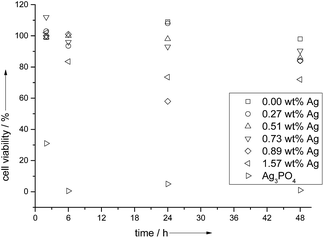
The cell viability assays (Fig. 10) with HeLa cells showed a 100% viability after 2 and 6 h incubation, but a subsequent drop in cell viability (to 58%) for silver concentrations at and above 0.89 wt% Ag. The pure calcium phosphate did not show a cytotoxicity, and in the presence of pure silver phosphate, all cells were dead after 6 h. This illustrates the delicate interplay between a bactericidal effect and a cytotoxic effect in the case of silver.6
Experimental part
A batch synthesis of silver-doped calcium phosphate was carried out by rapidly mixing solutions of Ca(NO3)2 (16.2 mM), (NH4)2HPO4 (10.8 mM) and AgNO3 (1.8 mM) and stirring at room temperature. Aliquots of the precipitate were taken from time to time and analysed with respect to their composition.The silver-doped calcium phosphate was precipitated in a continuous process26 by rapidly mixing equal volumes of the following aqueous solutions: 18 to 16.2 mmol L−1 Ca(NO3)2, 10.8 mmol L−1 of (NH4)2HPO4 and 0 to 1.8 mmol L−1 AgNO3. All solutions were prepared in ultrapure water. The pH of the calcium and phosphate solutions was adjusted to 9 with aqueous NH3 solution. After mixing the solutions for 48 s (hydrodynamic residence time in the precipitation vessel), the precipitated silver-doped calcium phosphate were filtered (residence time in the filter about 30 min) and subsequently dried at 70 °C. Silver phosphate was prepared under the same conditions. For the precipitation of silver phosphate, we used 30 mmol L−1 silver nitrate and 10 mmol L−1 diammonium hydrogen phosphate.
Thermogravimetry was carried out with a Netzsch STA 209 TG-DTA instrument. The samples were heated with 1 K min−1 under dynamic argon atmosphere (50 mL min−1) from 30 to 1400 °C. X-ray powder diffraction (XRD) was carried out with a Bruker D8 Advance instrument, operating with Cu Kα radiation (λ = 1.5406 Å). Scanning electron microscopy was carried out with an ESEM Quanta 400 FEG (FEI) instrument on gold–palladium (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20)-sputtered samples. Calcium and silver were determined by atomic absorption spectroscopy (AAS; Thermo Electron Corporation, M-Series AA spectrometer). Orthophosphate was determined by colorimetry with the molybdenum blue method (λ = 725 nm). IR spectra were recorded in KBr pellets with a Bruker Vertex 70 instrument.
20)-sputtered samples. Calcium and silver were determined by atomic absorption spectroscopy (AAS; Thermo Electron Corporation, M-Series AA spectrometer). Orthophosphate was determined by colorimetry with the molybdenum blue method (λ = 725 nm). IR spectra were recorded in KBr pellets with a Bruker Vertex 70 instrument.
For the silver release experiments, cylindrical pellets weighing 200 mg were prepared in an infrared press at 10 t for 15 min (diameter 11 mm). The pellets were put into a dialysis tube together with 5 mL ultrapure water. The external volume of the dialysis was 500 mL. The dialysis tube had a MWCO of 105 Da. The dialysis setups were kept at ambient temperature under continuous stirring with a magnetic stirrer under normal light exposure. During up to 152 days (3648 h), aliquots of 10 mL were taken and analyzed by AAS.
An Escherichia coli pre-culture in late exponential growth phase was diluted 1000-fold (10−3) in fresh LB broth medium (2% (w/v), Sigma-Aldrich). 100 μL of this diluted cell suspension was plated on an LB-agar plate (2% (w/v) LB broth, 1.5% (w/v) agar–agar). After 2 min incubation at room temperature, the different pellets (HA pellets with a silver content of 1.78 wt% and 0.88 wt% silver, silver phosphate and pure HA as control; weight about 200 mg each) were applied onto the inoculated LB-agar plate. The formation of inhibition zones was documented after overnight incubation of the LB-agar plates at 37 °C.
HeLa cells (human epithelial cervical cancer cells) were cultured in DMEM (Dulbecco's Modified Eagle's Medium), supplemented with 10% fetal calf serum (FCS) at 37 °C and 10% CO2 and subcultivated according to standard cell culture protocols. 24 h prior to the viability assay, the cells were trypsinised and seeded in cell culture dishes with 5 × 104 cells per well in 0.5 mL DMEM with FCS.
The cell viability was analyzed by an MTT assay 2 h, 6 h, 24 h and 48 h after the incubation with silver-doped calcium phosphate ceramics (one quarter of an 11 mm pellet, weighing originally 200 mg and after cutting into 4 parts weighing 50 mg, into each well) which contained 0 wt%, 0.27 wt%, 0.51 wt%, 0.73 wt%, 0.89 wt% and 1.57 wt% silver. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma, Taufkirchen, Germany) was dissolved in PBS (5 mg mL−1) and then diluted to 1 mg mL−1 in the cell culture medium. The cell culture medium of the incubated cells was replaced by 300 μL of the MTT solution and incubated for 1 h at 37 °C under 5% CO2 in humidified atmosphere. Then, the solution of MTT was replaced by 300 μL of DMSO. 30 min later, 100 μL aliquots were taken for spectrophotometric analysis with a Multiscan FC instrument (Thermo Fischer Scientific, Germany) at λ = 570 nm. The absorption of incubated cells was normalized to that of control (untreated) cells, thereby indicating the relative level of cell viability.
Conclusions
A continuous synthesis for poorly crystalline silver-doped calcium phosphate ceramics at ambient temperature with different silver content and their characterisation was developed which can be easily scaled up, giving reproducible silver contents in a nanocrystalline calcium phosphate ceramic. The presence of light strongly accelerates the uptake of silver into the calcium phosphate ceramics, leading to an in situ photoreduction to elemental silver, probably in the form of very small nanoparticles that were not detectable by X-ray diffraction. In all cases, a poorly crystalline apatite is obtained without peaks for silver phosphate or any other silver-containing crystalline phases. Antimicrobial studies with E. coli show a clear antimicrobial effect of pelleted samples. The silver-doped calcium phosphate ceramics do not show a significant effect of the viability of HeLa cells after 24 h below a silver concentration of 0.89 wt%. In contrast, stoichiometric silver phosphate has a high bactericidal effect due to a high release rate of silver ions, but this leads to a cytotoxic effect to eukaryotic cells as well, making silver phosphate unsuitable as a silver-containing ceramic material.In conclusion, the incorporation of silver into calcium phosphate ceramics has a potential to create a bactericidal biomaterial, but the incorporation of silver must be strictly controlled during the synthesis as this influences the silver release kinetics, and silver is also toxic to eukaryotic cells at higher concentrations.
Acknowledgements
We thank the Mercator foundation for support with a Mercur startup grant (to B.S. and M.E.).Notes and references
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130–3146 CrossRef CAS.
- M. Bohner, Mater. Today, 2010, 13, 24–30 CrossRef CAS.
- D. Arcos, A. R. Boccaccini, M. Bohner, A. Diez-Perez, M. Epple, E. Gomez-Barrena, A. Herrera, J. A. Planell, L. Rodriguez-Manas and M. Vallet-Regi, Acta Biomater., 2014, 10, 1793–1805 CrossRef CAS PubMed.
- S. Weiner and H. D. Wagner, Annu. Rev. Mater. Sci., 1998, 28, 271–298 CrossRef CAS.
- J. W. C. Dunlop and P. Fratzl, Annu. Rev. Mater. Sci., 2010, 40, 1–24 CrossRef CAS PubMed.
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed., 2013, 52, 1636–1653 CrossRef CAS PubMed.
- A. Peetsch, C. Greulich, D. Braun, C. Stroetges, H. Rehage, B. Siebers, M. Koeller and M. Epple, Colloids Surf., B, 2013, 102, 724–729 CrossRef CAS PubMed.
- K. S. Hwang, S. Hwangbo and J. T. Kim, J. Nanopart. Res., 2008, 10, 1337–1341 CrossRef CAS.
- Y. Zhang, Q. S. Yin, C. S. Zhou, H. Xia, Y. Zhang and Y. P. Jiao, J. Mater. Sci.: Mater. Med., 2014, 25, 801–812 CrossRef CAS PubMed.
- D. S. Syromotina, M. A. Surmeneva, S. N. Gorodzha, V. F. Pichugin, A. A. Ivanova, I. Y. Grubova, K. S. Kravchuk, K. V. Gogolinskii, O. Prymak, M. Epple and R. A. Surmenev, Russ. Phys. J., 2014, 56, 1198–1205 CrossRef CAS.
- A. Rajendran, R. C. Barik, D. Natarajan, M. S. Kiran and D. K. Pattanayak, Ceram. Int., 2014, 40, 10831–10838 CrossRef CAS PubMed.
- S. Jadalannagari, K. Deshmukh, S. R. Ramanan and M. Kowshik, Appl. Nanosci., 2014, 4, 133–141 CrossRef CAS PubMed.
- A. Dubnika and V. Zalite, Ceram. Int., 2014, 40, 9923–9930 CrossRef CAS PubMed.
- G. Ciobanu, S. Ilisei and C. Luca, Mater. Sci. Eng., C, 2014, 35, 36–42 CrossRef CAS PubMed.
- P. N. Lim, E. Y. Teo, B. Ho, B. Y. Tay and E. S. Thian, J. Biomed. Mater. Res., Part A, 2013, 101, 2456–2464 CrossRef PubMed.
- C. Ciobanu, S. Iconaru, P. Le Coustumer, L. Constantin and D. Predoi, Nanoscale Res. Lett., 2012, 7, 324 CrossRef PubMed.
- A. Ewald, D. Hösel, S. Patel, L. M. Grover, J. E. Barralet and U. Gbureck, Acta Biomater., 2011, 7, 4064–4070 CrossRef CAS PubMed.
- M. Díaz, F. Barba, M. Miranda, F. Guitián, R. Torrecillas and J. S. Moya, J. Nanomater., 2009, 2009, 1–6 CrossRef PubMed.
- J. R. J. Delben, O. M. Pimentel, M. B. Coelho, P. D. Candelorio, L. N. Furini, F. Alencar dos Santos, F. S. de Vicente and A. A. S. T. Delben, J. Therm. Anal. Calorim., 2009, 97, 433–436 CrossRef CAS PubMed.
- L. Yang, X. Ning, Q. Xiao, K. Chen and H. Zhou, J. Biomed. Mater. Res., Part B, 2007, 81, 50–56 CrossRef PubMed.
- N. Rameshbabu, T. S. Sampath Kumar, T. G. Prabhakar, V. S. Sastry, K. V. G. K. Murty and K. Prasad Rao, J. Biomed. Mater. Res., Part A, 2007, 80, 581–591 CrossRef CAS PubMed.
- W. Chen, S. Oh, A. P. Ong, N. Oh, Y. Liu, H. S. Courtney, M. Appleford and J. L. Ong, J. Biomed. Mater. Res., Part A, 2007, 82, 899–906 CrossRef CAS PubMed.
- S. K. Arumugam, T. P. Sastry, B. Sreedhar and A. B. Mandal, J. Biomed. Mater. Res., Part A, 2007, 80, 391–398 CrossRef PubMed.
- S. M. F. Asmus, S. Sakakura and G. Pezzotti, J. Compos. Mater., 2003, 37, 2117–2129 CrossRef CAS PubMed.
- X. Zhang, G. H. M. Gubbels, R. A. Terpstra and R. Metselaar, J. Mater. Sci., 1997, 32, 235–243 CrossRef CAS.
- D. Tadic, F. Peters and M. Epple, Biomaterials, 2002, 23, 2553–2559 CrossRef CAS.
- C. S. Liu, Y. Huang, W. Shen and J. H. Cui, Biomaterials, 2001, 22, 301–306 CrossRef CAS.
- Z. Zyman, A. Goncharenko, D. Rokhmistrov and M. Epple, Materialwiss. Werkstofftech., 2011, 42, 154–157 CrossRef CAS PubMed.
- L. Badrour, A. Sadel, M. Zahir, L. Kimakh and A. El Hajbi, Annales de Chimie Science des Matériaux, 1998, 23, 61–64 CrossRef CAS.
- D. Tadic and M. Epple, Biomaterials, 2004, 25, 987–994 CrossRef CAS.
- H. Yang, B. Xiao and K. W. Xu, J. Mater. Sci.: Mater. Med., 2009, 20, 785–792 CrossRef CAS PubMed.
- M. Thomas, S. K. Ghosh and K. C. George, Mater. Lett., 2002, 56, 386–392 CrossRef CAS.
- K. Loza, J. Diendorf, C. Greulich, L. Ruiz-Gonzales, J. M. Gonzalez-Calbet, M. Vallet-Regi, M. Koeller and M. Epple, J. Mater. Chem. B, 2014, 2, 1634–1643 RSC.
- S. Kittler, C. Greulich, J. Diendorf, M. Köller and M. Epple, Chem. Mater., 2010, 22, 4548–4554 CrossRef CAS.
- D. R. Lide, CRC Handbook of Chemistry and Physics, Internet Version, 2005 Search PubMed.
- C. Greulich, D. Braun, A. Peetsch, J. Diendorf, B. Siebers, M. Epple and M. Koller, RSC Adv., 2012, 2, 6981–6987 RSC.
| This journal is © The Royal Society of Chemistry 2015 |