Air-stable ambipolar organic field-effect transistors based on naphthalenediimide–diketopyrrolopyrrole copolymers†
Ping Wanga,
Hui Lib,
Chunling Gub,
Huanli Dongb,
Zhenzhen Xua and
Hongbing Fu*ab
aBeijing Key Laboratory for Optical Materials and Photonic Devices, Department of Chemistry, Capital Normal University, Beijing 100048, P. R. China. E-mail: hongbing.fu@iccas.ac.cn; Fax: +86 10 6890 7107; Tel: +86 10 6890 7107
bBeijing National Laboratory for Molecular Sciences (BNLMS), Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. Fax: +86-10-82616517; Tel: +86-10-62526801
First published on 9th February 2015
Abstract
Two air-stable polymeric semiconductors were rationally designed and synthesized, namely PNDI-DPP and PNDI-T(DPP)T, containing naphthalenediimide (NDI) units and diketopyrrolopyrrole (DPP). The coplanar thiophene-substituted DPP moieties act as donors rather than acceptors, even though DPP is an electron-deficient core. In a bottom-gate/top-contact device architecture, the effect of changing the number of thiophene linkers on the performance of the two completely different OFETs was investigated. PNDI-T(DPP)T presented unipolar p-type behaviour with an average hole mobility of 0.02 cm2 V−1 s−1, while PNDI-DPP exhibited ambipolar transport with average electron and hole mobilities of 5.7 × 10−3 and 1.6 × 10−3 cm2 V−1 s−1, respectively. Moreover, OFETs based on the two polymers showed good air-stability with negligible changes after being stored under ambient conditions for over 3 months.
Introduction
Polymeric semiconductors are very popular in organic field-effect transistors (OFETs) because they have the following advantages: designability and tailorability, functional diversity, low cost and the availability of a wide variety of sources.1–4 High performance OFETs are mostly fabricated using a single-component layer of donor–acceptor (D–A) conjugated polymers,5 because of strong interchain interaction and large π-delocalization. In recent years, the progress in p-type D–A polymers as active layers for OFETs is notable, with mobilities surpassing 10 cm2 V−1 s−1. However, in order to achieve applications in complementary-like circuits,6–8 and light-emitting transistors,9–12 etc., the development of ambipolar OFETs is particularly important. So far, most of the high performance ambipolar OFETs are obtained under nitrogen rather than in ambient conditions. Therefore, the development of air-stable ambipolar polymeric semiconductors is an urgent requirement. To achieve ambipolar transport, one of the requirements is that the LUMO energy levels of the D–A polymers are low enough. The fine-tuning of energy levels is realized through the appropriate combination of donor and acceptor moieties to achieve efficient electron and hole transport.13–15 On this basis, appropriate selection of electron-rich and electron-deficient components is a key step in order to obtain an ideal ambipolar semiconductor.16,17 In addition, the introduction of appropriate alkyl side chains also plays an important role, not only increasing the solubility of polymers to result in facile solution processing but also influencing intermolecular interactions and molecular packing.18,19Diketopyrrolopyrrole (DPP) core is excellent structural unit for the D–A polymers which have been demonstrated high hole or electron transporting motilities in thin-film devices.20–24 Ambipolar DPP-based co-polymers have been reported by Yang group25–27 and Sirringhaus group,28–30 exhibiting high performance even with balanced hole and electron mobilities, and mostly are applied to complementary metal oxide semiconductor (CMOS), exhibiting high switching speed and excellent signal robustness. However, in these reported works, DPP is generally regarded as an acceptor moiety. DPP as the donor moiety is not universally investigated in D–A polymers.
In our article, the D–A polymers were designed and synthesized by combining NDI core and DPP core through thiophene (T) linkers, namely PNDI-DPP and PNDI-T(DPP)T. The polymer of PNDI-DPP exhibited air-stable and ambipolar transport with electron and hole mobilities up to 5.7 × 10−3 ± 0.001 cm2 V−1 s−1 and 1.6 × 10−3 ± 0.002 cm2 V−1 s−1, respectively. Compared with PNDI-DPP, PNDI-T(DPP)T only presented unipolar p-type with hole mobility up to 0.02 ± 0.007 cm2 V−1 s−1. As we all know, DPP core is electron deficient group and generally acts as acceptor in D–A polymers. However, thiophene-substituted DPP moieties serve as donors in our work illustrated by Density functional theory calculations. The decrease of thiophene linkers from 2 to 1 in the polymeric backbone of PNDI-mT(DPP)mT (m = 0, 1) leads to the LUMO energy level down from 3.95 to 4.18. In addition, the ambipolar PNDI-DPP OFETs were integrated into organic complementary inverters, resulting in effective inversion characteristics with gains of ∼8.6 as a result of balanced transport properties. Microstructures of both polymers were investigated by two dimensional grazing incidence X-ray diffraction (2D-GIXRD) to gain a further understanding of the charge transport properties.
Experimental section
Materials
All the starting materials were purchased from Aldrich, Alfa, Acros, or TCI and used directly without further purification. All the common reagents and solvents were obtained from Beijing Chemical Works. Toluene was distilled from sodium under an argon atmosphere, and benzophenone was acted as the indicator.Instruments
NMR spectra were recorded on a Bruker ANACE 400 MHz spectrometer in CDCl3 solutions with tetramethylsilane (TMS) (TMS; δ = 0 ppm) as internal standard. Mass spectra were obtained on a Bruker Daltonics BIFLEX III MALDI-TOF analyzer using MALDI mode. Molecular weights of polymers was determined by gel permeation chromatography (GPC) with a Waters 515 chromatograph connected to a Waters 2410 refractive index detector, using THF as eluent and polystyrene standards as calibrants. Thermogravimetric analysis (TGA) measurements were performed on Shimadzu thermogravimetric analyzer (model DTG-60) under a nitrogen flow with a heating rate of 10 °C min−1. Differential scanning calorimetry (DSC) analyses were carried out with a METTLER TOLEDO Instrument DSC822 calorimeter. UV-vis absorption spectra were measured on a Shimidazu UV-3600 UV-vis-NIR spectrophotometer. Cyclic voltammograms were obtained by an electrochemistry workstation with a CHI voltammetric analyzer at a scan rate of 50 mV s−1. The conventional three-electrode configuration consisted of a platinum working electrode coated with a thin film layer of polymer, a platinum wire auxiliary electrode, and an Ag/AgCl reference electrode with ferrocene–ferrocenium (Fc+–Fc) as the internal standard; the absolute energy level of ferrocene–ferrocenium is 4.8 eV below vacuum. Tetrabutylammonium hexafluorophosphate (TBAPF6) (0.1 M) was used as the supporting electrolyte. DFT calculations are performed at the B3LYP/6-31G level. For grazing incidence X-ray diffraction (GIXRD), the spin-coated films were illuminated at a constant incidence angle of 0.2° (λ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 1.54 Å).
θ = 1.54 Å).
Device fabrication and characterization
OFET devices were manufactured with a top-contact bottom-gate structure. An n-type heavily doped Si wafer with 300 nm thick thermally grown SiO2 (Ci = 10 nF cm−2) was used as gate electrode and dielectric layer. The Si/SiO2 substrate was cleaned carefully by pure water, ethanol, acetone, and a mixed solution of concentrated sulfuric acid and hydrogen peroxide in 140 °C, and then treated with octadecyltrichlorosilane (OTS) to form a self-assembled monolayer. Polymer films (40 ± 3 nm) were spin-coated from Chlorobenzene solution with a concentration of 8 mg mL−1 and subsequently annealed for 30 min under nitrogen. Gold source and drain electrodes (60 nm) were deposited through vacuum evaporation on the organic layer using a shadow mask. The channel length (L) and width (W) were 200 μm and 500 μm, respectively. CMOS-like inverters were fabricated on a substrate with larger channel dimensions (W = 500 μm and L = 30 μm). Electrical characterization was performed on a Keithley 4200 SCS (semiconductor characterization system) with a micromanipulator 6150 probe station under an ambient atmosphere for the OFETs and inverter characterizations. The mobility of the OFETs in the saturation region was extracted from the equation IDS = Ciμ(W/2L) (VG − VT)2, where IDS is the drain current, Ci is the capacitance per unit area of the SiO2 dielectric layer (Ci = 10 nF cm−2), μ is field-effect carrier mobility, W and L are the channel width and length, VG is the gate voltage, and VT is the threshold voltage. The mobility data were collected from more than seven different devices.Syntheses of the monomers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v) as eluent, obtaining a dark red solid as the product 3 (2.36 g, yield 15%). 1H NMR (400 MHz, CDCl3): δ = 8.88 (d, 2H), 7.61 (d, 2H), 7.25 (d, 2H), 4.01 (m, 4H), 1.85 (d, 2H), 1.35–1.23 (m, 17H), 0.87–0.85 (m, 13H). 13C NMR (100 MHz, CDCl3): δ = 161.8, 140.5, 135.4130.6, 129.9, 128.5, 108.0, 45.9, 39.2, 30.3, 28.4, 23.6, 23.1, 14.1, 10.6. MALDI-TOF-MS [M]+: 524.6 (m/z).
100, v/v) as eluent, obtaining a dark red solid as the product 3 (2.36 g, yield 15%). 1H NMR (400 MHz, CDCl3): δ = 8.88 (d, 2H), 7.61 (d, 2H), 7.25 (d, 2H), 4.01 (m, 4H), 1.85 (d, 2H), 1.35–1.23 (m, 17H), 0.87–0.85 (m, 13H). 13C NMR (100 MHz, CDCl3): δ = 161.8, 140.5, 135.4130.6, 129.9, 128.5, 108.0, 45.9, 39.2, 30.3, 28.4, 23.6, 23.1, 14.1, 10.6. MALDI-TOF-MS [M]+: 524.6 (m/z).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v). The pure product 4 was collected as a purple solid (1.69 g, 83% yield). 1H NMR (400 MHz, CDCl3): δ = 8.63 (d, 2H), 7.22 (d, 2H), 3.89 (m, 4H), 1.83 (d, 2H), 1.34–1.25 (m, 19H), 0.90–0.84 (m, 13H). MALDI-TOF-MS [M]+: 682.0 (m/z).
10, v/v). The pure product 4 was collected as a purple solid (1.69 g, 83% yield). 1H NMR (400 MHz, CDCl3): δ = 8.63 (d, 2H), 7.22 (d, 2H), 3.89 (m, 4H), 1.83 (d, 2H), 1.34–1.25 (m, 19H), 0.90–0.84 (m, 13H). MALDI-TOF-MS [M]+: 682.0 (m/z).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to get the final product 5 as a mazarine solid (158 mg, 23% yield). 1H NMR (400 MHz, CDCl3): δ = 8.92 (s, 2H), 7.31 (d, 6H), 7.06 (t, 2H), 4.10–3.99 (m, 4H), 1.92 (d, 2H), 1.38–1.27 (m, 18H), 0.93–0.85 (m, 13H). MALDI-TOF-MS [M]+: 688.0 (m/z).
5, v/v) to get the final product 5 as a mazarine solid (158 mg, 23% yield). 1H NMR (400 MHz, CDCl3): δ = 8.92 (s, 2H), 7.31 (d, 6H), 7.06 (t, 2H), 4.10–3.99 (m, 4H), 1.92 (d, 2H), 1.38–1.27 (m, 18H), 0.93–0.85 (m, 13H). MALDI-TOF-MS [M]+: 688.0 (m/z).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v). The pure product 6 was collected as a black solid (277 mg, 82% yield). 1H NMR (400 MHz, CDCl3): δ = 8.86 (s, 2H), 7.24 (d, 2H), 7.03 (d, 4H), 4.07–3.97 (m, 4H), 1.89 (s, 2H), 1.39–1.26 (m, 16H), 0.92–0.85 (m, 12H). MALDI-TOF-MS [M]+: 845.9 (m/z).
8, v/v). The pure product 6 was collected as a black solid (277 mg, 82% yield). 1H NMR (400 MHz, CDCl3): δ = 8.86 (s, 2H), 7.24 (d, 2H), 7.03 (d, 4H), 4.07–3.97 (m, 4H), 1.89 (s, 2H), 1.39–1.26 (m, 16H), 0.92–0.85 (m, 12H). MALDI-TOF-MS [M]+: 845.9 (m/z).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, v/v) as eluent to give a yellow solid 10 (1.9 g, 19%). 1H NMR (400 MHz, CDCl3): δ = 8.99 (s, 2H), 4.13 (d, 4H), 2.00–1.96 (m, 2H), 1.30–1.23 (m, 64H), 0.89–0.83 (m, 13H).
150, v/v) as eluent to give a yellow solid 10 (1.9 g, 19%). 1H NMR (400 MHz, CDCl3): δ = 8.99 (s, 2H), 4.13 (d, 4H), 2.00–1.96 (m, 2H), 1.30–1.23 (m, 64H), 0.89–0.83 (m, 13H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, v/v). The purified product 11 (610 mg, 87%) was slightly yellow liquid. 1H NMR (400 MHz, CDCl3): δ = 8.89 (t, 2H), 4.11 (d, 4H), 1.96 (s, 2H), 1.56–1.48 (m, 14H), 1.36–1.18 (m, 96H), 0.89–0.81 (m, 33H).
150, v/v). The purified product 11 (610 mg, 87%) was slightly yellow liquid. 1H NMR (400 MHz, CDCl3): δ = 8.89 (t, 2H), 4.11 (d, 4H), 1.96 (s, 2H), 1.56–1.48 (m, 14H), 1.36–1.18 (m, 96H), 0.89–0.81 (m, 33H).Syntheses of the polymers
Results and discussion
Synthesis and thermal stability
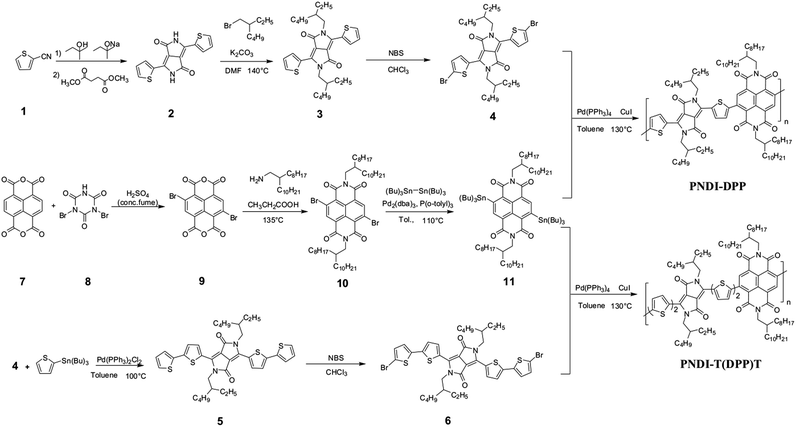
The synthesis of PNDI-DPP and PNDI-T(DPP)T is depicted in Scheme 1. Compound 2 was started from thiophene-2-carbonitrile, followed by alkylation and dibrominated to give monomer 4.33 Compound 5 was synthesized by Stille coupling of compound 4 with tributyl(thiophen-2-yl)stannane, and then bromized with N-bromosuccinimide to get the monomer 6. The polymers PNDI-DPP, PNDI-T(DPP)T were synthesized via Stille coupling between compound 11 and monomer 4, and monomer 6, respectively. Both polymers were purified by Soxhlet extraction with methanol, acetone, and hexane and finally collected from chloroform. PNDI-DPP and PNDI-T(DPP)T were soluble in chloro-solvents, such as chloroform and chlorobenzene.The molecular weights of both polymers were evaluated by gel permeation chromatography (GPC) with THF solution as eluent relative to polystyrene standard. The number average molecular weight (Mn) of PNDI-DPP was 16.6 kDa, and its weight average molecular weight (Mw) was 20.8 kDa. For PNDI-T(DPP)T, it had a relatively lower Mn of 9.7 kDa and Mw (16.5 kDa). The lower molecule weight of PNDI-T(DPP)T is probably due to its poor solubility in THF. Both polymers showed narrow polydispersity index (PDI: PNDI-DPP, 1.1; PNDI-T(DPP)T, 1.3). To determine the thermal properties of polymers, thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis were performed under nitrogen. Both polymers exhibited excellent thermal stability: PNDI-DPP with 5% weight loss at 409.0 °C and PNDI-T(DPP)T with 5% weight loss at 396.9 °C. DSC thermographs of polymers did not reveal obvious endothermic or exothermic peaks in the range of 0–300 °C, suggesting no evidence of melting or crystallization transitions.
Optical properties
The UV-vis absorption spectra of both polymers were measured in chloroform solutions (ca. 10−6 M) and in thin films (Fig. 1). In solutions, the absorption spectra of both polymers show the single and structureless bands, with the maximum at 750 nm for PNDI-DPP and 663 nm for PNDI-T(DPP)T. Remarkably, the absorption band of PNDI-T(DPP)T is obviously blue-shifted relative to that of PNDI-DPP due to the introduction of thiophene units. This blue-shift is likely to be the result of steric effect and will be discussed in following part. In solid state, both the 0–0 peaks and the 0–1 peaks of polymers show red-shifts and the enhanced, which are attributed to solid-state packing effects.34 The PNDI-DPP film shows dual absorption bands, with the 0–0 vibrational peak at 906 nm and 0–1 vibrational peak at 834 nm. The absorption spectrum of PNDI-T(DPP)T film presents the 0–0 vibrational peak at 709 nm and 0–1 vibrational peak at 660 nm. Furthermore, the optical energy bandgap estimated from the absorption onsets in thin films are 1.15 eV for PNDI-DPP and 1.25 eV for PNDI-T(DPP)T. The increase in the energy gap for PNDI-T(DPP)T compared with PNDI-DPP is different from other polymers whose spectra would red-shift when incorporation more thiophene units. This is probably because the large torsion configuration of polymer chains in PNDI-T(DPP)T which reduce the efficient conjugation length.35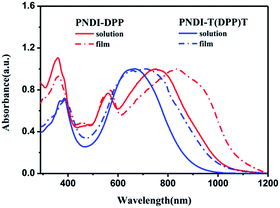 | ||
| Fig. 1 Normalized absorption spectra of both polymers in solution (chloroform) and in thin films spin-coated on glass. | ||
DFT-calculated molecular geometries and orbitals
To predict the minimum-energy conformation of the polymer backbone and the frontier orbital energy levels (LUMO/HOMO), quantum-chemical calculations (B3LYP/6-31G) were performed for the trimmers of both polymers. Long alkyl chains were replaced by methyl groups in order to reduce the calculation time. This treatment does not affect the results because the influence of alkyl chain on the electronic structure of the polymers can be ignored.36 As shown in Fig. 2, the dihedral angle between NDI core and the neighboring thiophene units is about 44° in the trimmers of PNDI-DPP, which is much smaller than that of PNDI-T(DPP)T (about 64°). A more intuitive diagram of molecular conformations is showed in Fig. S5 in the (ESI†). From this, it can be seen that the introduction of thiophene units to the polymer backbone reduces the planarity of the PNDI-T(DPP)T configuration and impairs delocalization along polymer chains. The result is consistent with the fact that blue-shift spectra are observed both in solution and film of PNDI-T(DPP)T compared with those of PNDI-DPP. The HOMO orbitals of both polymers are mainly delocalized over the DPP moieties, and the LUMO orbitals are mainly located in NDI parts. In both polymers, thiophene-substituted DPP moieties serve as donors, though DPP is generally considered as the electron-deficient core.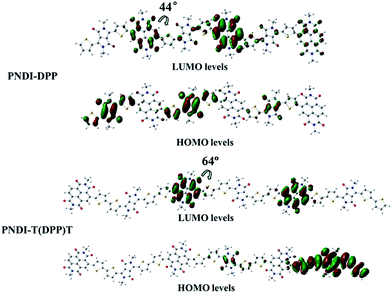 | ||
| Fig. 2 LUMO/HOMO orbitals of the trimmers of PNDI-DPP and PNDI-T(DPP)T obtained with DFT-calculations (B3LYP/6-31G). | ||
Electrochemical properties
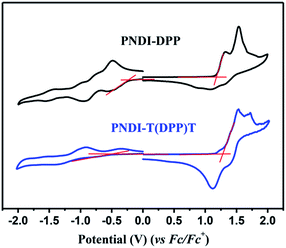
The electrochemical properties of both polymers are investigated by cyclic voltammetry (CV). The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) were estimated through this measurement. As depicted in Fig. 3, for PNDI-DPP, the intensity of reduction peak is approximately same with the oxidation peak. However, the oxidation peak of PNDI-T(DPP)T is stronger than the reduction peak. It is worth mentioning that both the oxidation peaks and reduction peaks for both polymers are reversible. The HOMO and LUMO values are calculated from the oxidation and reduction onsets of CV curves, and all data are summarized in Table 1. The HOMO value of −5.60 eV and LUMO value of −4.18 eV for PNDI-DPP just locate in the energy ranges that ensure the injection of both hole and electron from the Au electrodes.37 Whereas, the HOMO and LUMO levels of polymer PNDI-T(DPP)T are estimated to be −5.55/−3.95 eV, indicating that PNDI-T(DPP)T is possibly more suitable for the p-type semiconductor. The electrochemical energy bandgaps of polymers are 1.42 and 1.60 eV, respectively, larger than the optical bandgaps. The LUMO levels of both polymers are low enough for achieving air-stable charge transport.38| Polymers | λmax/onseta (nm) | Eoptgb (eV) | ECVHOMOc (eV) | ECVLUMOc (eV) | ECVgd (eV) |
|---|---|---|---|---|---|
a Maximum absorption and absorption onset in thin film.b Optical band gap estimated from the onset of film absorption.c Electrochemically determined vs. Fc/Fc+, 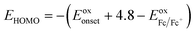 , , 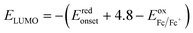 .d Energy band gap calculated from the difference between EHOMO and ELUMO. .d Energy band gap calculated from the difference between EHOMO and ELUMO. |
|||||
| PNDI-DPP | 834, 906/1079 | 1.15 | −5.60 | −4.18 | 1.42 |
| PNDI-T(DPP)T | 660, 709/995 | 1.25 | −5.55 | −3.95 | 1.60 |
Characteristics of organic field-effect transistors
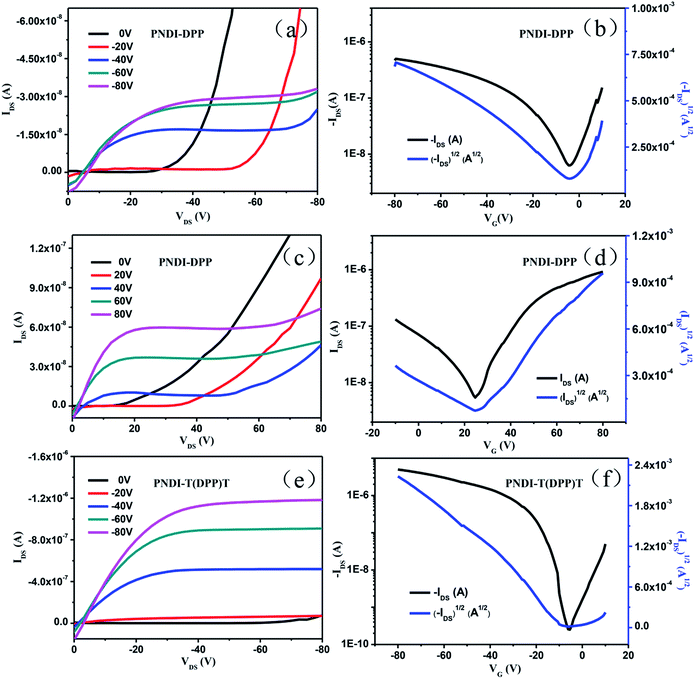
To test the transistor performance of both polymers, typical bottom-gate/top-contact devices with a channel length of 200 μm and a width of 500 μm were fabricated by spin-coating polymer solutions (8 mg mL−1 in chlorobenzene) onto octadecyltrichlorosilance (OTS) treated SiO2 (300 nm)/n++-Si substrates. After thermal annealing at optimized temperature of 160 °C for 30 min under nitrogen, 60 nm thick gold electrodes were deposited on the polymer films through a shadow mask. Fig. 4 shows the typical output and transfer curves of the OFETs tested in ambient air conditions without any special precautions, and the performance data are listed in Table 2. It can be obviously observed that OFET devices based on PNDI-T(DPP)T exhibit unipolar p-type transport characteristics while PNDI-DPP devices show balanced ambipolar transport characteristics. Calculated at saturated region, PNDI-T(DPP)T presents an hole mobility of 0.02 cm2 V−1 s−1, while PNDI-DPP exhibits the electron and hole mobilities of 5.7 × 10−3 (μe) and 1.6 × 10−3 (μh) cm2 V−1 s−1, respectively.A transition from ambipolar (both hole and electron transport) for PNDI-DPP to p-channel (hole transport) for PNDI-T(DPP)T can be attributed to increasing the thiophene units from 1 to 2 in the polymer backbone. This indicated that the thiophene units have a profound effect on their charge carrier transport behavior. Additionally, the OFET devices of both polymers show good stability. For instance, even though stored in ambient for 3 months without encapsulation, the charge mobilities of both polymers were almost unchanged. OFETs transfer curves for fresh devices and after 3 months storage in ambient based on polymers were showed in Fig. S6.†
Characteristics of complementary inverters
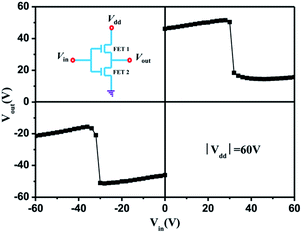
The balanced carrier mobilities of polymer PNDI-DPP was observed in OFETs, therefore, CMOS-like inverters based on two identical ambipolar PNDI-DPP OFETs (W = 500 μm, L = 30 μm) were fabricated using a common gate electrode as the input voltage (Vin). The output voltage (Vout) was monitored as a function of Vin at constant supply bias (Vdd). A efficiently inversion of Vout from low voltage about ±14 V to high voltage about ±50 V is shown in Fig. 5 with Vdd = ± 60 V. The steepness of the inverter curves indicate that PNDI-DPP ambipolar semiconductors yield the voltage gains (−dVout/dVin) of ∼8.6. The inversion of Vin is observed at about ±30 V, half of the control Vdd, which is ideal for ambipolar inverters.39Microstructural study of the polymer thin films
The morphology and crystallinity of polymer films were key parameters for device performance. The thin film microstructures of both polymers were studied using grazing incidence X-ray diffraction (GIXRD). The two-dimensional GIXRD intensity profiles of the polymer thin films are shown in Fig. 6. For polymer thin film of PNDI-T(DPP)T, a strong diffraction peak at 2θ = 4.5° is observed in the out-of-plane pattern and ascribed to (100) diffraction of lamellar structure, corresponding to a chain-to-chain distance of 19.6 Å. The (200) diffraction peak also appears, indicating long-range order in PNDI-T(DPP)T. In the in-plane pattern, the lack of obvious peaks imply that there is no π–π stacking. These results suggest that PNDI-T(DPP)T may solely adopt the edge-on packing model in thin films. Compared with PNDI-T(DPP)T, PNDI-DPP exhibits different molecular packing. For PNDI-DPP thin film, the (100) diffraction peaks are observed not only in the out-of-plane pattern at 2θ = 4.3° but also in the in-plane pattern, corresponding to a interlayer distance of 20.5 Å. Thus, the thin film of PNDI-DPP has not just an edge-on configuration, but also a face-on configuration. These findings reveal that the molecular arrangement of PNDI-T(DPP)T is more ordered than PNDI-DPP in the thin films. The edge-on packing would be more conducive to charge carrier transport throughout the polymer film,40,41 which may be the reason of higher hole mobilities of PNDI-T(DPP)T than that of PNDI-DPP. AFM height images of polymer thin films after annealing at 160 °C for 30 min are showed in Fig. S7.† The intertwined polymer nanofibers were observed for both PNDI-DPP and PNDI-T(DPP)T.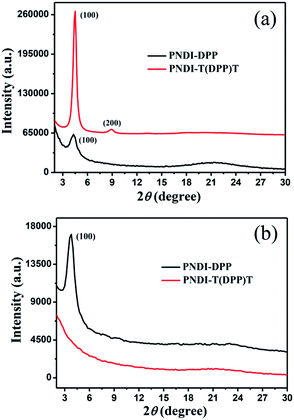 | ||
| Fig. 6 (a) Out of plane X-ray diffractograms and (b) in-plane grazing incidence X-ray diffractograms of polymer films annealed at 160 °C. | ||
Conclusions
We have designed, synthesized and characterized two donor–acceptor polymers PNDI-DPP and PNDI-T(DPP)T. DFT calculation results showed that the thiophene-substituted DPP moieties served as donors. Unipolar p-type transport (μh = 0.02 cm2 V−1 s−1) was observed for OFETs based on PNDI-T(DPP)T. By simply reducing the thiophene in the repeat unit, balanced ambipolarity was achieved in PNDI-DPP under ambient condition with electron and hole mobilities of 5.7 × 10−3 and 1.6 × 10−3 cm2 V−1 s−1, respectively. Our results give an example that a transition from unipolar to ambipolar transport could be achieved through adjusting number of thiophene units in the backbone, and demonstrated an efficient approach to achieve balanced hole and electron mobilities of NDI-based co-polymer, which is applied in CMOS-like circuits.Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 21073200, 21273251, 91333111, 21190034, 21221002), Beijing Municipal Science & Technology Commission (no. Z131103002813097), project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140512), the National Basic Research Program of China (973) 2011CB808402, 2013CB933500, and the Chinese Academy of Sciences. The GIXRD data were obtained at 1W1A, Beijing Synchrotron Radiation Facility. The authors gratefully acknowledge the assistance of scientists of the Diffuse X-ray Scattering Station during the experiments.Notes and references
- H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dotz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679 CrossRef CAS PubMed.
- H. Sirringhaus, Science, 2000, 290, 2123 CrossRef CAS.
- J. H. Schön, S. Berg, C. Kloc and B. Batlogg, Science, 2000, 287, 1022 CrossRef.
- Z. Bao, W. K. Chan and L. Yu, J. Am. Chem. Soc., 1995, 117, 12426 CrossRef CAS.
- M. Muccini, Nat. Mater., 2006, 5, 605 CrossRef CAS PubMed.
- J. C. Bijleveld, A. P. Zoombelt, S. G. J. Mathijssen, M. M. Wienk, M. Turbiez, D. M. de Leeuw and R. A. J. Janssen, J. Am. Chem. Soc., 2009, 131, 16616 CrossRef CAS PubMed.
- J. Fan, J. D. Yuen, M. F. Wang, J. Seifter, J. H. Seo, A. R. Mohebbi, D. Zakhidov, A. Heeger and F. Wudl, Adv. Mater., 2012, 24, 2186 CrossRef CAS PubMed.
- M. L. Tang, A. D. Reichardt, N. Miyaki, R. M. Stoltenberg and Z. Bao, J. Am. Chem. Soc., 2008, 130, 6064 CrossRef CAS PubMed.
- J. Zaumseil, C. L. Donley, J. S. Kim, R. H. Friend and H. Sirringhaus, Adv. Mater., 2006, 18, 2708 CrossRef CAS.
- L. Bürgi, M. Turbiez, R. Pfeiffer, F. Bienewald, H.-J. Kirner and C. Winnewisser, Adv. Mater., 2008, 20, 2217 CrossRef.
- S. N. Bhat, R. D. Pietro and H. Sirringhaus, Chem. Mater., 2012, 24, 4060 CrossRef CAS.
- M. C. Gwinner, D. Kabra, M. Roberts, T. J. K. Brenner, B. H. Wallikewitz, C. R. McNeill, R. H. Friend and H. Sirringhaus, Adv. Mater., 2012, 24, 2728 CrossRef CAS PubMed.
- P. Sonar, S. P. Singh, Y. Li, M. S. Soh and A. Dodabalapur, Adv. Mater., 2010, 22, 5409 CrossRef CAS PubMed.
- A. Luzio, D. Fazzi, D. Natali, E. Giussani, K.-J. Baeg, Z. Chen, Y.-Y. Noh, A. Facchetti and M. Caironi, Adv. Funct. Mater., 2014, 24, 1151 CrossRef CAS.
- M. M. Szumilo, E. H. Gann, C. R. McNeill, V. Lemaur, Y. Oliver, L. Thomsen, Y. Vaynzof, M. Sommer and H. Sirringhaus, Chem. Mater., 2014, 26, 6796 CrossRef CAS.
- J. Wang, X. Chen, Z. Cai, H. Luo, Y. Li, Z. Liu, G. Zhang and D. Zhang, Polym. Chem., 2013, 4, 5283 RSC.
- J. D. Yuen, J. Fan, J. Seifter, B. Lim, R. Hufschmid, A. J. Heeger and F. Wudl, J. Am. Chem. Soc., 2011, 133, 20799 CrossRef CAS PubMed.
- J. Mei, D. H. Kim, A. L. Ayzner, M. F. Toney and Z. Bao, J. Am. Chem. Soc., 2011, 133, 20130 CrossRef CAS PubMed.
- J. D. Yuen and F. Wudl, Energy Environ. Sci., 2013, 6, 392 CAS.
- J. D. Yuen, R. Kumar, D. Zakhidov, J. Seifter, B. Lim, A. J. Heeger and F. Wudl, Adv. Mater., 2011, 23, 3780 CAS.
- X. Zhang, L. J. Richter, D. M. DeLongchamp, R. J. Kline, M. R. Hammond, I. McCulloch, M. Heeney, R. S. Ashraf, J. N. Smith, T. D. Anthopoulos, B. Schroeder, Y. H. Geerts, D. A. Fischer and M. F. Toney, J. Am. Chem. Soc., 2011, 133, 15073 CrossRef CAS PubMed.
- P.-T. Wu, F. S. Kim and S. A. Jenekhe, Chem. Mater., 2011, 23, 4618 CrossRef CAS.
- B. Sun, W. Hong, H. Aziz and Y. Li, Polym. Chem., 2015, 6, 938 RSC.
- C. Kanimozhi, N. Yaacobi-Gross, K. W. Chou, A. Amassian, T. D. Anthopoulos and S. Patil, J. Am. Chem. Soc., 2012, 134, 16532 CrossRef CAS PubMed.
- S. Cho, J. Lee, M. Tong, J. H. Seo and C. Yang, Adv. Funct. Mater., 2011, 21, 1910 CrossRef CAS.
- J. Lee, A. R. Han, J. Kim, Y. Kim, J. H. Oh and C. Yang, J. Am. Chem. Soc., 2012, 134, 20713 CrossRef CAS PubMed.
- J. Lee, A. R. Han, H. Yu, T. J. Shin, C. Yang and J. H. Oh, J. Am. Chem. Soc., 2013, 135, 9540 CrossRef CAS PubMed.
- Z. Chen, M. J. Lee, R. Shahid Ashraf, Y. Gu, S. Albert-Seifried, M. Meedom Nielsen, B. Schroeder, T. D. Anthopoulos, M. Heeney, I. McCulloch and H. Sirringhaus, Adv. Mater., 2012, 24, 647 CrossRef CAS PubMed.
- A. J. Kronemeijer, E. Gili, M. Shahid, J. Rivnay, A. Salleo, M. Heeney and H. Sirringhaus, Adv. Mater., 2012, 24, 1558 CrossRef CAS PubMed.
- M. Shahid, R. S. Ashraf, Z. Huang, A. J. Kronemeijer, T. McCarthy-Ward, I. McCulloch, J. R. Durrant, H. Sirringhaus and M. Heeney, J. Mater. Chem., 2012, 22, 12817 RSC.
- A. D. Lebsack, J. T. Link, L. E. Overman and B. A. Stearns, J. Am. Chem. Soc., 2002, 124, 9008 CrossRef CAS PubMed.
- L. S. Liebeskind and R. W. Fengl, J. Org. Chem., 1990, 55, 5359 CrossRef CAS.
- C. H. Woo, P. M. Beaujuge, T. W. Holcombe, O. P. Lee and J. M. J. Fréchet, J. Am. Chem. Soc., 2010, 132, 15547 CrossRef CAS PubMed.
- H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. Shakya Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Wienk, R. A. J. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272 CrossRef CAS PubMed.
- H. Li, C. Gu, L. Jiang, L. Wei, W. Hu and H. Fu, J. Mater. Chem. C, 2013, 1, 2021 RSC.
- L. Tan, Y. Guo, G. Zhang, Y. Yang, D. Zhang, G. Yu, W. Xu and Y. Liu, J. Mater. Chem., 2011, 21, 18042 RSC.
- J. L. Kan, Y. L. Chen, D. D. Qi, Y. Q. Liu and J. Z. Jiang, Adv. Mater., 2012, 24, 1755 CrossRef CAS PubMed.
- C. Gu, W. Hu, J. Yao and H. Fu, Chem. Mater., 2013, 25, 2178 CrossRef CAS.
- Y. Zhang, C. Kim, J. Lin and T.-Q. Nguyen, Adv. Funct. Mater., 2012, 22, 97 CrossRef CAS.
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, B. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herwig and D. M. de Leeuw, Nature, 1999, 401, 685 CrossRef CAS PubMed.
- J. E. Donaghey, E.-H. Sohn, R. S. Ashraf, T. D. Anthopoulos, S. E. Watkins, K. Song, C. K. Williams and I. McCulloch, Polym. Chem., 2013, 4, 3537 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: GPC, TGA, DSC, 2D-GIXRD pattern of polymers. See DOI: 10.1039/c5ra00391a |
| This journal is © The Royal Society of Chemistry 2015 |