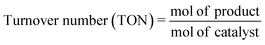Solid state synthesis, characterization, optical properties and cooperative catalytic performance of bismuth vanadate nanocatalyst for Biginelli reactions
Shahin Khademiniaa,
Mahdi Behzad*a and
Hamideh Samari Jahromib
aDepartment of Chemistry, Semnan University, Semnan 35351-19111, Iran. E-mail: mbehzad@semnan.ac.ir; mahdibehzad@gmail.com; Tel: +98 233 338 3195
bResearch Institute of Petroleum Industry (RIPI), Environment and Biotechnology Division, West Blvd., Azadi Sports Complex, P.O. Box 14665-1998, Tehran, Iran
First published on 27th February 2015
Abstract
Bi2V2O7 nano powders were synthesized via a solid state reaction at 500 °C for 8 h using Bi(NO3)3 and VO(acac)2 at stoichiometric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Bi
1 Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V molar ratio as raw materials. The synthesized material was characterized by the powder X-ray diffraction (PXRD) technique and Fourier-transform infrared (FTIR) spectroscopy. Structural analysis was performed by the FullProf program employing profile matching with constant scale factors. The results showed that the pattern had a main Bi2V2O7 structure with a space group of Fd
V molar ratio as raw materials. The synthesized material was characterized by the powder X-ray diffraction (PXRD) technique and Fourier-transform infrared (FTIR) spectroscopy. Structural analysis was performed by the FullProf program employing profile matching with constant scale factors. The results showed that the pattern had a main Bi2V2O7 structure with a space group of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. Lattice parameters were found as a = b = c = 10.259079 Å, α = γ = β = 90°. FESEM and TEM images showed that the synthesized Bi2V2O7 particles had mono-shaped sphere morphologies. Ultraviolet-visible spectrum analysis showed that the nanostructured Bi2V2O7 powders possessed strong light absorption properties in the ultraviolet light region. Catalytic performance of the synthesized nanomaterial was also investigated in Biginelli reactions which showed excellent efficiency.
m. Lattice parameters were found as a = b = c = 10.259079 Å, α = γ = β = 90°. FESEM and TEM images showed that the synthesized Bi2V2O7 particles had mono-shaped sphere morphologies. Ultraviolet-visible spectrum analysis showed that the nanostructured Bi2V2O7 powders possessed strong light absorption properties in the ultraviolet light region. Catalytic performance of the synthesized nanomaterial was also investigated in Biginelli reactions which showed excellent efficiency.
1. Introduction
Among oxides and fluorides with general formula A2B2X7 (where A is a medium-large cation and B is an octahedrally coordinated, high-charge cation), there are many compounds widely studied for their great technological properties.1,2 The pyrochlore phase is understood as a defect structure of fluorite type with general formula A2B2O6 or A2B2O7. This structure exhibits ccp arrays of cations with anions partially occupying the tetrahedral sites to form a distorted structure containing intrinsic defects. Research on pyrochlore materials have been focused on applications such as catalysis,3,4 electronics,5 optical6 and magnetic properties7 and solid oxide fuel cell (SOFC) electrode materials.8 Bi-containing perovskites have received a lot of attention as lead-free ferroelectric9,10 and multi-ferroic11,12 materials. Among them, bismuth vanadate semiconductor materials have also received great interest for their photocatalytic application for splitting of water into hydrogen and oxygen13–16 and organic wastewater treatment.17–19 Several compounds of Bi–V–O have been previously synthesized via different methods including BiVO3.2,20 BiVO3, BiVO4, Bi4V2O10.5, Bi1.62V8O16,21 different phases of Bi2VO5.5,22 Bi4V2O11 and Bi4V2O10,23 Bi4V2O11,24 Bi2VO5.5 and so on.25The Biginelli reaction, which was originally reported by Biginelli in 1891,26 is a methodology for the synthesis of 3,4-dihydropyrimidin-2-(1H)-one derivatives (DHPMs) in a one-step procedure. DHPMs have already shown biological activities.27 Several metal oxides have been reported as nanocatalysts for the Biginelli reactions including alumina supported Mo catalysts,28 nano ZnO as a structure base catalyst,29 MoO3–ZrO2 nanocomposite,30 MnO2–MWCNT nanocomposites,31 TiO2 nanoparticles,32 Mg–Al–CO3 and Ca–Al–CO3 hydrotalcite,33 Bi2O3/ZrO2 nanocomposite,34 ZrO2–Al2O3–Fe3O4,35 imidazole functionalized Fe3O4@SiO2,36 alumina supported MoO3,37 ZrO2-pillared clay,38 ZnO nanoparticle,39 Fe3O4–CNT,40 TiO2–MWCNT,41 Fe3O4@mesoporous SBA-15.42 In the present study, a solid state method was explored for the synthesis of nanostructured Bi2V2O7 powders using Bi(NO3)3 and VO(acac)2 as raw materials. To the best of our knowledge, there is no information available in the literature about the solid state synthesis of nanostructured Bi2V2O7 crystallites. The band gap energy of the as-prepared Bi2V2O7 nanomaterial was initially estimated from ultraviolet-visible spectra. Catalytic application of the synthesized Bi2V2O7 was also investigated in Biginelli reactions. It was found that the synthesized Bi2V2O7 nanocatalyst had excellent efficiency in the synthesis of DHPMs.
2. Experimental
2.1. General remarks
All chemicals were of analytical grade, obtained from commercial sources, and used without further purification. Phase identifications were performed on a powder X-ray diffractometer D5000 (Siemens AG, Munich, Germany) using CuKα radiation. The morphology of the obtained materials was examined with a field emission scanning electron microscope (Hitachi FE-SEM model S-4160). Bi2V2O7 particles were dispersed in water and cast onto a copper grid to study the sizes and morphology of the particles by TEM (Transmission Electron Microscopy) using a Philips – CM300 – 150 kV microscope. From the TEM images the average of particle size distribution was carried out using Image software. FTIR spectra were recorded on a Tensor 27 (Bruker Corporation, Germany). Absorption spectra were recorded on an Analytik Jena Specord 40 (Analytik Jena AG Analytical Instrumentation, Jena, Germany). BET surface areas were acquired on a Beckman Coulter SA3100 Surface Area Analyser. The purity of products was checked by thin layer chromatography (TLC) on glass plates coated with silica gel 60 F254 using n-hexane/ethyl acetate mixture as mobile phase.2.2. Catalyst preparation
In a typical synthetic experiment, 0.50 g (1.03 mmol) of Bi(NO3)3 (Mw = 485.08 g mol−1) and 0.27 g (1.03 mmol) of VO(acac)2 (Mw = 262.14 g mol−1) were mixed in a mortar and ground until a nearly homogeneous powder was obtained. The obtained powder was added into a 25 mL crucible and treated thermally in one step at 500 °C for 8 h. The crucible was then cooled normally in oven to the room temperature. The obtained powder was collected for further analyses. The synthesis yield for Bi2V2O7 (Mw = 631.88 g mol−1) was 0.30 g (92%).2.3. General procedure for the synthesis of DHPMs
In a typical procedure, a mixture of aldehyde (1 mmol), ethyl acetoacetate (1 mmol), urea (1.2 mmol) and 0.02 g (3.1 × 10−2 mmol) of Bi2V2O7 as catalyst were placed in a round-bottom flask under solvent free conditions. The suspension was stirred at 90 °C. The reaction was monitored by thin layer chromatography (TLC) [6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 hexane
4 hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate]. After completion of the reaction, the solid crude product was washed with deionized water to separate the unreacted raw materials. The precipitated solid was then collected and dissolved in ethanol to separate the solid catalyst. The filtrate was left undisturbed at room temperature to afford the crystals of the pure product.
ethylacetate]. After completion of the reaction, the solid crude product was washed with deionized water to separate the unreacted raw materials. The precipitated solid was then collected and dissolved in ethanol to separate the solid catalyst. The filtrate was left undisturbed at room temperature to afford the crystals of the pure product.
3. Result and discussion
3.1. Characterizations
The phase composition of the Bi2V2O7 nanomaterial was examined by powder X-ray diffraction technique. Fig. 1 shows the PXRD pattern of the obtained material in the 2θ range 4–70°. The results of structural analysis performed by the FullProf program employing profile matching with constant scale factors are also included in Fig. 1. Red bars are the observed intensities while the black ones are the calculated data. The blue one is the difference: Yobs − Ycalc. The Bragg reflections positions are indicated by green bars. The pattern has a well fitted defect pyrochlore structure profile with face centered cubic structure. Lattice parameters were found as a = b = c = 10.259079 Å and α = γ = β = 90° with a space group of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.20 The results showed that the pattern had a main Bi2V2O7 structure. A small fraction of unreacted V2O3 was found in the structure which is identified in Fig. 1.43
m.20 The results showed that the pattern had a main Bi2V2O7 structure. A small fraction of unreacted V2O3 was found in the structure which is identified in Fig. 1.43
 | ||
| Fig. 1 PXRD pattern of the synthesized Bi2V2O7 nanomaterial and the rietveld analysis. ◊ is due to V2O3 impurity. | ||
Fig. 2 shows the FESEM images of the synthesized Bi2V2O7 nanomaterial. Fig. 3 also shows the TEM images as well as the particle size distribution profile. As could be seen from Fig. 2 and 3, the materials are consisted of nearly homogeneous narrow size distributed spherical particles. Fig. 3 also indicates that the maximum particle size distribution were in the range of 40–60 nm.
UV-vis absorption spectrum of the Bi2V2O7 nanomaterial is shown in Fig. 4a. The optical band gap is also shown in Fig. 4b. The pure Bi2V2O7 nanomaterial displays a typical visible absorption edge at about 720 nm. According to the results of Pascual et al.,44 the relation between the absorption coefficient and incident photon energy can be written as (αhν)2 = A(hν − Eg), where A and Eg are constant and direct band gap energy, respectively. Band gap energy was evaluated by extrapolating the linear part of the curve to the energy axis. It was found that the band gap was 2.0 eV.45,46
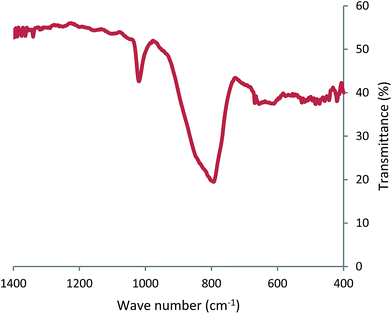
Fig. 5 shows the FTIR spectrum of the obtained material. There are some peaks at around 420, 472, 516, 611, 655, 794, 1020, 1097 and 1151 cm−1 that are the characteristic bands for Bi2V2O7. The band at 420 cm−1 is attributed to Bi–O vibration;47,48 the bands at 516–611 and 655 cm−1 (ref. 24) are attributed to the O–V–O deformation mode vibration. The broad band at about 794–950 cm−1 is assigned to the V–O asymmetric stretching vibration.25,47–49 The signal at around 1020 cm−1 is also assigned to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. This band is usually observed in vanadium oxide compounds with intermediate oxidation state between V5+ and V4+ ions.50 The peak at around 601 cm−1 is assigned to the Bi–O stretching vibration and the peak at around 1090 cm−1 is assigned to the Bi–O vibrations caused by the interaction between the Bi–O bonds and their surroundings.51,52 The surface area, average pore size and average pore volume of the synthesized material was analyzed by BET and BJH textural analyses. The sample was degassed at 150 °C for 120 min in the nitrogen atmosphere prior to N2-physical adsorption measurements. The specific surface area (SBET) of the obtained materials was determined with adsorption–desorption isotherms of N2 at 77 K. The surface area, pore volumes and average pore diameters of the synthesized materials are summarized in Table 1.53
O stretching vibration. This band is usually observed in vanadium oxide compounds with intermediate oxidation state between V5+ and V4+ ions.50 The peak at around 601 cm−1 is assigned to the Bi–O stretching vibration and the peak at around 1090 cm−1 is assigned to the Bi–O vibrations caused by the interaction between the Bi–O bonds and their surroundings.51,52 The surface area, average pore size and average pore volume of the synthesized material was analyzed by BET and BJH textural analyses. The sample was degassed at 150 °C for 120 min in the nitrogen atmosphere prior to N2-physical adsorption measurements. The specific surface area (SBET) of the obtained materials was determined with adsorption–desorption isotherms of N2 at 77 K. The surface area, pore volumes and average pore diameters of the synthesized materials are summarized in Table 1.53
| SBET (m2 g−1) | Pore size (Å) | Pore volume (cm3 g−1) |
|---|---|---|
| 3.0 | 139.6 | 0.01 |
3.2. Catalytic studies
| Amount of catalyst (g) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|
a Benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetoacetate ethylacetoacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea molar ratios is as follows: 1 urea molar ratios is as follows: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. 1.2. |
|||
| 0.01 | 60 | 90 | 43 |
| 0.02 | 60 | 90 | 89 |
| 0.03 | 60 | 90 | 89 |
| 0.04 | 60 | 90 | 89 |
| 0.05 | 60 | 90 | 89 |
| 0.02 | 60 | 60 | 58 |
| 0.02 | 60 | 70 | 77 |
| 0.02 | 60 | 80 | 81 |
| 0.02 | 60 | 90 | 89 |
| 0.02 | 60 | 100 | 89 |
| 0.02 | 15 | 90 | 50 |
| 0.02 | 30 | 90 | 66 |
| 0.02 | 45 | 90 | 81 |
| 0.02 | 60 | 90 | 89 |
| 0.02 | 90 | 90 | 89 |
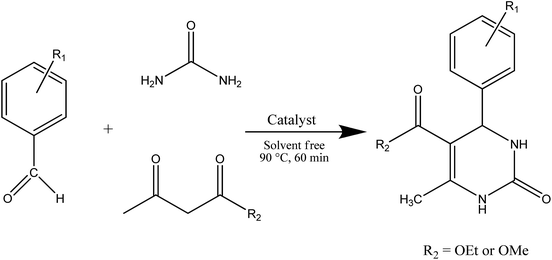
The optimized parameters from the previous section were used for the synthesis of other derivatives and the results are collected in Table 3. Scheme 1 shows a summary of the reaction pathway.
| R1 | R2 | mmol of product | Turnover number (TON) |
|---|---|---|---|
| H | OEt | 0.89 | 29 |
| 4-Cl | OEt | 0.92 | 30 |
| 2-Cl | OEt | 0.98 | 32 |
| 4-Br | OEt | 0.62 | 20 |
| 4-F | OEt | 0.69 | 22 |
| 2-OMe | OEt | 0.98 | 32 |
| 4-OMe | OEt | 0.72 | 23 |
| 4-Cl | OMe | 0.98 | 32 |
| 2-Cl | OMe | 0.90 | 29 |
| 4-Br | OMe | 0.93 | 30 |
| 4-F | OMe | 0.98 | 32 |
| 2-OMe | OMe | 0.92 | 30 |
| 4-OMe | OMe | 0.62 | 20 |
The turnover numbers (TON, mol of product per mol of catalyst) for the synthesis of DHPMs were calculated by the following equation.54
Table 4 shows the catalytic efficiency of the synthesized Bi2V2O7 nanomaterial compared to the starting materials Bi(NO3)3 and VO(acac)2. The optimized conditions from the previous section were used. As could be seen from Table 4, Bi2V2O7 was much more efficient catalyst compared to the two starting materials which mean that the presence of the both metal ions was important and the two ions have acted cooperatively.
| Catalyst | Reagents | Time (min) | Yield |
|---|---|---|---|
| Bi2V2O7 | Benzaldehyde | 60 | 89 |
| VO(acac)2 | Benzaldehyde | 60 | 38 |
| Bi(NO3)3 | Benzaldehyde | 60 | 35 |
To show the merit of the present work, we have compared Bi2V2O7 nanocatalyst results with some of the previously reported catalysts in the synthesis of DHPMs (Table 5). It is clear that Bi2V2O7 showed greater activity than some other heterogeneous catalysts.
| Catalyst | R1 | Catalyst amount | Condition | Yield% | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Bi2V2O7 | H | 3.1 × 10−2 mmol | Solvent-free, 90 °C | 89 | 60 | This work |
| 4-Cl | 92 | |||||
| 2-Cl | 98 | |||||
| Bi2O3/ZrO2 | H | 20 mol% | Solvent-free, 80–85 °C | 85 | 120 | 34 |
| 4-Cl | 85 | 120 | ||||
| 2-Cl | 82 | 165 | ||||
| ZrO2–Al2O3–Fe3O4 | H | 0.05 g | Ethanol, reflux, 140 °C | 82 | 300 | 35 |
| 4-Cl | 66 | |||||
| 2-Cl | 40 | |||||
| Mo/γ-Al2O3 | H | 0.3 g | Solvent-free conditions at 100 °C | 80 | 60 | 37 |
| ZnO | H | 25 mol% | Solvent-free conditions at 90 °C | 92 | 50 | 39 |
| 4-Cl | 95 |
4. Conclusion
In this work, Bi2V2O7 nanomaterial was synthesized via solid state method. PXRD patterns and structural analysis done by the FullProf program employing profile matching showed that the synthesis was successfull. FESEM and TEM images showed the sphere-like structure in the as-synthesized materials. The catalytic application of the synthesized nanomaterial was investigated in Biginelli reaction in solvent free conditions. It was found that Bi2V2O7 nanomaterial had excellent efficiency in the synthesis of DHPMs. Besides, the two metal ions have performed the catalytic reactions cooperatively.References
- J. B. Thomson, A. R. Armstrong and P. G. Bruce, J. Solid State Chem., 1999, 148, 56 CrossRef CAS.
- H. Kishimoto, T. Omata, S. Otsuka-Yao-Matsuo and K. Ueda, J. Alloys Compd., 2000, 312, 94 CrossRef CAS.
- D. J. Haynes, D. A. Berry, D. Shekhawat and J. J. Spivey, Catal. Today, 2008, 136, 206 CrossRef CAS PubMed.
- R. Kieffer, M. Fujiwara, L. Udron and Y. Souma, Catal. Today, 1997, 36, 15 CrossRef CAS.
- K. Matsuhira, M. Wakeshima, Y. Hinatsu and S. Takagi, J. Phys. Soc. Jpn., 2011, 80 DOI:10.1143/JPSJ.80.094701.
- M. G. Brik, A. M. Srivastava and N. M. Avram, Opt. Mater., 2011, 33, 1671 CrossRef CAS PubMed.
- K. A. Ross, L. R. Yaraskavitch, M. Laver, J. S. Gardner, J. A. Quilliam, S. Meng, J. B. Kycia, D. K. Singh, T. Proffen, H. A. Dabkowska and B. D. Gaulin, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 174442 CrossRef.
- J. K. Gill, O. P. Pandey and K. Singh, Solid State Sci., 2011, 13, 1960 CrossRef CAS PubMed.
- N. Izyumskaya, Y. Alivov and H. Morkoc, Crit. Rev. Solid State Mater. Sci., 2009, 34, 89 CrossRef CAS.
- J. Rodel, W. Jo, K. T. P. Seifert, E. M. Anton, T. Granzow and D. Damjanovic, J. Am. Ceram. Soc., 2009, 92, 1153 CrossRef PubMed.
- R. Ramesh and N. A. Spaldin, Nat. Mater., 2007, 6, 21 CrossRef CAS PubMed.
- N. A. Hill, J. Phys. Chem. B, 2000, 104, 6694 CrossRef CAS.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- A. Fujishima, X. T. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS PubMed.
- H. Tada, M. Fujishima and H. Kobayashi, Chem. Soc. Rev., 2011, 40, 4232 RSC.
- Z. G. Zou, J. H. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625 CrossRef CAS PubMed.
- A. Molinari, R. Argazzi and A. Maldotti, J. Mol. Catal. A: Chem., 2013, 372, 23 CrossRef CAS PubMed.
- X. V. Doorslaer, K. Demeestere, P. M. Heynderickx, M. Caussyn, H. V. Langenhove, F. Devlieghere and A. Vermeulen, Appl. Catal., B, 2013, 138, 333 CrossRef PubMed.
- L. Z. Pei, S. Wang, Y. K. Xie, H. Y. Yu and Y. H. Guo, J. Alloys Compd., 2013, 587, 625 CrossRef PubMed.
- N. Ramadass, T. Palanisamy, J. Gopalakrishnan, G. Aravamudan and M. V. C. Sastri, Solid State Commun., 1975, 17, 545 CrossRef CAS.
- M. Dragomir and M. Valant, Ceram. Int., 2013, 39, 5963 CrossRef CAS PubMed.
- P. Millán, J. M. Rojo and A. Castro, Mater. Res. Bull., 2000, 35, 835 CrossRef.
- S. Sorokina, R. Enjalbert, P. Baules, A. Castro and J. Galy, J. Solid State Chem., 1999, 144, 379 CrossRef CAS.
- S. Beg and N. A. S. Al-Areqi, Philos. Mag., 2009, 89, 1279 CrossRef CAS.
- S. Beg, S. Hafeez and N. A. S. Al-Areqi, Philos. Mag., 2010, 90, 4579 CrossRef CAS.
- P. Biginelli, Ber. Dtsch. Chem. Ges., 1891, 24, 1317 CrossRef.
- K. Singh, D. Arora and S. Singh, Mini-Rev. Med. Chem., 2009, 9, 95 CrossRef CAS.
- K. Kouachi, G. Lafaye, S. Pronier, L. Bennini and S. Menad, J. Mol. Catal. A: Chem., 2014, 395, 210 CrossRef CAS PubMed.
- F. Tamaddon and S. Moradi, J. Mol. Catal. A: Chem., 2013, 370, 117 CrossRef CAS PubMed.
- S. Samantaray and B. G. Mishra, J. Mol. Catal. A: Chem., 2011, 339, 92 CrossRef CAS PubMed.
- J. Safari and S. G. Ravandi, J. Mol. Catal. A: Chem., 2013, 373, 72 CrossRef CAS PubMed.
- H. R. Memarain and M. Ranjbar, J. Mol. Catal. A: Chem., 2012, 356, 46 CrossRef CAS PubMed.
- J. Lal, M. Sharma, S. Gupta, P. Parashar, P. Sahu and D. D. Agarwal, J. Mol. Catal. A: Chem., 2012, 352, 31 CrossRef CAS PubMed.
- V. C. Guguloth, G. Raju, M. Basude and S. Battu, Int. J. Environ. Anal. Chem., 2014, 5, 86 Search PubMed.
- A. Wang, X. Liu, Z. Su and H. Jing, Catal. Sci. Technol., 2014, 4, 71 CAS.
- J. Javidi, M. Esmaeilpour and F. N. Dodeji, RSC Adv., 2015, 5, 308 RSC.
- S. L. Jain, V. V. D. N. Prasad and B. Sain, Catal. Commun., 2008, 9, 499 CrossRef CAS PubMed.
- V. Singh, V. Sapehiyia, V. Srivastava and S. Kaur, Catal. Commun., 2006, 7, 571 CrossRef CAS PubMed.
- K. Pourshamsian, Int. J. Nano Dimens., 2015, 6, 99 Search PubMed.
- J. Safari and S. G. Ravandi, RSC Adv., 2014, 4, 11486 RSC.
- J. Safari and S. G. Ravandi, New J. Chem., 2014, 38, 3514 RSC.
- J. Mondal, T. Sen and A. Bhaumik, Dalton Trans., 2012, 41, 6173 RSC.
- H. Fei, X. Ding, M. Wei and K. Wei, Solid State Sci., 2011, 13, 2049 CrossRef CAS PubMed.
- J. Pascual, J. Camassel and M. Mathieu, Phys. Rev. B: Solid State, 1978, 18, 5606 CrossRef CAS.
- N. A. S. Al-Areqi, A. S. N. Al-Kamali, K. A. S. Ghaleb, A. Al-Alas and K. Al-Mureish, Radiat. Eff. Defects Solids, 2014, 169, 117 CrossRef CAS.
- B. Qi, L. Wu, Y. Zhang, Q. Zeng and J. Zhi, J. Colloid Interface Sci., 2010, 345, 181 CrossRef CAS PubMed.
- N. A. S. Al-Areqi, S. Beg and A. Al-Alas, J. Phys. Chem. Solids, 2012, 73, 730 CrossRef CAS PubMed.
- S. Beg, S. Hafeez and N. A. S. Al-Areqi, Defect Diffus. Forum, 2011, 316, 7 CrossRef PubMed.
- S. Beg, S. Hafeez and N. A. S. Al-Areqi, Phys. B (Amsterdam, Neth.), 2010, 405, 4370 CrossRef CAS PubMed.
- F. J. Quites and H. O. Pastore, Mater. Res. Bull., 2010, 45, 892 CrossRef CAS PubMed.
- Y. Sun, W. Wang, L. Zhang and Z. Zhang, Chem. Eng. J. (Amsterdam, Neth.), 2012, 211, 161 Search PubMed.
- L. Zhang, W. Wang, J. Yang, Z. Chen, W. Zhang, L. Zhou and S. Liu, Appl. Catal., A, 2006, 308, 105 CrossRef CAS PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2002, 41, 4538 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |