Enhanced mechanical properties of ammonia-modified graphene nanosheets/epoxy nanocomposites
Dong-Dong Zhang,
Dong-Lin Zhao*,
Ran-Ran Yao and
Wei-Gang Xie
State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers (Beijing University of Chemical Technology), Ministry of Education, Beijing Engineering Research Center of Environmental Material for Water Purification, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: dlzhao@mail.buct.edu.cn; Fax: +86 10 64434914; Tel: +86 10 64434914
First published on 11th March 2015
Abstract
Ammonia-modified graphene nanosheets (AMGNSs)/epoxy nanocomposites were prepared by using a facile blend method. Graphene nanosheets (GNSs) were modified with aqueous ammonia (NH3·H2O) and hydrogen peroxide (H2O2), to obtain amine (–NH2) functionalized GNSs and to enhance the bonding between the GNSs and epoxy resin matrix. The effects of the AMGNSs on the static and dynamic mechanical properties of the nanocomposites were investigated. The results indicated that the tensile and flexural strength and modulus of the AMGNS/epoxy nanocomposites first increased and then decreased with increasing addition of AMGNSs. The addition of 0.5 wt% AMGNSs improved the tensile strength and flexural modulus of the pristine epoxy by 27.84% and 7.75%, respectively. Meanwhile, the addition of 0.1 wt% AMGNSs improved the tensile modulus and flexural strength of the pristine epoxy by 14.16% and 94.38%, respectively. The reinforcing effect of the AMGNSs in enhancing the impact properties of the epoxy nanocomposites was also examined. It was demonstrated that the amine-functionalization of GNSs with ammonia had an obvious effect on the mechanical performances of epoxy matrix nanocomposites.
1. Introduction
Graphene, a single-layer carbon sheet of sp2-hybridized carbon atoms arranged in a hexagonal packed lattice structure, has shown many remarkable properties, such as excellent electronic transport properties, high specific surface area, high thermal conductivity and extraordinary mechanical properties.1–8 In fact, carbon-based fillers (carbon black, carbon nanotubes, etc.) have been extensively researched for reinforcement of polymer nanocomposites for the past decade.9 Carbon nanotubes, as the strongest contender of graphene in the nanocomposites field, are not ideal for toughening or reinforcing polymer composites because of high viscosity, prohibitively high cost, and high anisotropic functionality.9,10 As a new allotrope of elemental carbon, graphene has been used as a new carbon-based filler for polymer nanocomposites to obtain remarkable physical and mechanical properties.11–17Epoxy resin systems are important thermoset materials used as adhesives, coatings, structural materials, insulating materials, and for many other industrial applications at present.9 Graphene-reinforced epoxy resin nanocomposites have been investigated in view of their enhanced mechanical and thermal properties.6,9,18–26 However, the bad dispersion homogeneity due to the high specific surface area of graphene and the weak interfacial interactions between graphene and epoxy resin restrict the application of graphene in polymer nanocomposites.27–29 It is likely that the proper chemical modification of graphene can prevent the aggregation of graphene sheets as well as improve the interfacial interactions between graphene and epoxy resin and improve the processability.16,30,31
In this study, we focused on the homogeneous dispersion of graphene and the changes in mechanical properties with its content increasing. As previously studied for carbon-based fillers, dispersion and interfacial interactions are two main issues governing the properties of nanocomposites.20 Graphene obtained from the reduction of graphene oxide contains carboxylic functional groups its edges, which could provide active sites to react with aqueous ammonia, and the amine (–NH2) functionalization of graphene nanosheets (GNSs) could obviously enhance the interfacial adhesion between GNSs and epoxy resin.19,32 Herein, GNSs were surface-treated with aqueous ammonia (NH3·H2O) and hydrogen peroxide (H2O2, 30%) to obtain amine functionalized GNSs and enhance the bonding between the GNSs and epoxy resin matrix. Meanwhile, the effects of ammonia-modified GNSs (AMGNSs) on the static and dynamic mechanical properties and the freeze-fractured morphologies of AMGNS/epoxy nanocomposites were investigated.
2. Experimental
2.1 Materials
Natural flake graphite (320 mesh) was purchased from Dongxin Electrical Carbon Co. Ltd., China. Concentrated sulfuric acid (H2SO4, 95–98%), potassium permanganate (KMnO4) and concentrated hydrochloric acid (HCl, 36–38%) were all obtained from Beijing Yili Fine Chemical Co. Ltd. Hydrogen peroxide (H2O2, 30%), ammonia water (NH3·H2O) and sodium nitrate (NaNO3) were obtained from Beijing Chemical Works. Epoxy resin (E-51, WSR618) based on bisphenol-A with an epoxy value of 0.50–0.56 was obtained from Wuxi Resin Factory, China. The anhydride hardener (methyl tetrahydrophthalic anhydride, MeTHPA) and the accelerator (2,4,6-tris(dimethylaminomethyl)phenol, DMP-30) were obtained from Beijing Chem. Reagent Co., China.2.2 Preparation of GNSs
The modified Hummers method was conducted to oxidize natural flake graphite (320 mesh) for the synthesis of graphite oxide.33 Typically, 3.0 g of flake graphite, 1.5 g of NaNO3 and 69 ml of H2SO4 (95–98%) were mixed in an ice bath and then were stirred for 15 min utilizing a magnetic stirring apparatus. Subsequently, 9.0 g of KMnO4 was gradually added. The mixture was allowed to react at 35 °C for 60 min. Then, 138 ml of distilled water (DI water) was gradually added into the mixture within 10 min and the mixture was allowed to react at 98 °C for 15 min. Then, the reaction was terminated by adding 420 ml of DI water and 30 ml of 30% H2O2 solution, resulting in a yellow-brown mixture. The mixture was centrifuged at 4000 rpm for 5 min and washed at least three times with a solution of 5% HCl until sulphate could not be detected with BaCl2. The obtained graphite oxide deposits were dispersed in 500 ml of DI water utilizing a high-power ultrasonic machine for 1.5 h. Then the solution was centrifuged again at 4000 rpm for 5 min to eliminate insoluble substances. After centrifugation, 150 ml of HCl (36–38%) was added into the supernatant liquid and then the suspension was centrifuged again under the same conditions. After the graphite oxide was dried at 65 °C in the air for 24 h, it was placed into a ceramic crucible to be heated at 1050 °C for 30 s in a muffle furnace. Consequently, the resulting expanded graphite was dispersed in ethanol at a ratio of 0.2 g/100 ml, and then the suspension was sonicated for 15 h utilizing a high-power ultrasonic machine. Finally, after removing the redundant ethanol in the suspension, the mixture was heated at 120 °C for 4 h in a vacuum oven to get the GNSs.2.3 Preparation of AMGNSs
Briefly, 1.5 g GNSs was added into a 240 ml mixed solution of H2O2 (30%) and NH3·H2O at a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Then, the mixture was stirred at room temperature for 15 min. Consequently, the solution was treated under sonication for 4 h. After that, the resultant alkali-treated GNSs were filtered using a core funnel and washed at least three times with DI water until the solution was neutral. After filtration, the mixture was dried at 65 °C for 12 h in a vacuum oven to get the AMGNSs.
5. Then, the mixture was stirred at room temperature for 15 min. Consequently, the solution was treated under sonication for 4 h. After that, the resultant alkali-treated GNSs were filtered using a core funnel and washed at least three times with DI water until the solution was neutral. After filtration, the mixture was dried at 65 °C for 12 h in a vacuum oven to get the AMGNSs.
2.4 Preparation of AMGNS/epoxy nanocomposites
A weight ratio of epoxy to MeTHPA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 was used to prepare the AMGNS/epoxy nanocomposites. The AMGNSs were manually mixed with a mixture of epoxy and MeTHPA. Then, the suspension was treated under sonication for 3 h to make a homogeneous dispersion AMGNSs in the epoxy system. After a few drops of DMP-30 (accelerator) were added into the mixture, more than 30 min of ultrasonication was needed. The resultant suspension was degassed in a vacuum oven at 50 °C for 30 min to remove air bubbles. Consequently, the mixture was poured into a stainless steel mold to cure at 90 °C for 1 h, 130 °C for 2 h, and then 160 °C for 2 h. After curing, the nanocomposite samples were cooled naturally to room temperature. The AMGNS weight fractions of 0 wt%, 0.05 wt%, 0.1 wt%, 0.5 wt%, and 1 wt% were prepared.
0.7 was used to prepare the AMGNS/epoxy nanocomposites. The AMGNSs were manually mixed with a mixture of epoxy and MeTHPA. Then, the suspension was treated under sonication for 3 h to make a homogeneous dispersion AMGNSs in the epoxy system. After a few drops of DMP-30 (accelerator) were added into the mixture, more than 30 min of ultrasonication was needed. The resultant suspension was degassed in a vacuum oven at 50 °C for 30 min to remove air bubbles. Consequently, the mixture was poured into a stainless steel mold to cure at 90 °C for 1 h, 130 °C for 2 h, and then 160 °C for 2 h. After curing, the nanocomposite samples were cooled naturally to room temperature. The AMGNS weight fractions of 0 wt%, 0.05 wt%, 0.1 wt%, 0.5 wt%, and 1 wt% were prepared.
2.5 Characterization techniques and instruments
The morphologies of the GNSs and AMGNSs were investigated using transmission electron microscopy (TEM, Hitachi H-800). Fourier transform infrared (FT-IR) spectra of the GNSs and AMGNSs were recorded on a Nicolet Nexus 670 FT-IR spectrometer. Tensile and flexural tests were carried out with an Instron™ 1185 mechanical testing machine at room temperature with crosshead speeds of 10 mm min−1 and 5 mm min−1, respectively. The tensile specimens were a standard dumbbell-shape according to GB/T 1040-1992 (Fig. 1), and the geometric dimensions of the samples for flexural tests were 80 mm (length) × 15 mm (width) × 4 mm (thickness) according to GB/T 9341-2008 (Fig. 2). The freeze-fractured surfaces of the AMGNS/epoxy nanocomposites with different AMGNSs contents were observed using scanning electron microscopy (SEM, S-4700). The dynamic mechanical properties of the AMGNS/epoxy nanocomposites were performed on a dynamic mechanical analysis (DMA) VA3000 (01dB-Metravib Instruments Inc., France) at a frequency of 1 Hz, from 25 to 200 °C at a heating rate of 5 °C min−1. The geometric dimensions of the DMA samples were 50 mm (length) × 6 mm (width) × 2 mm (thickness). The Charpy impact strength of the samples was tested with a Resil Impactor 6957 (Ceast Instruments Inc., Italy) according to GB/T 2571-1995.3. Results and discussion
3.1 Characterization of GNSs and AMGNSs
The morphology and structure of the GNSs and AMGNSs were characterized using TEM. As shown in Fig. 3, the GNSs and AMGNSs consist of almost transparent carbon nanosheets with a thin wrinkled structure. Both the GNSs and AMGNSs are entangled with each other and resemble crumpled papers. The curled and corrugated structure is the intrinsic characteristic of graphene. It is well known that as-prepared GNSs still contain carboxylic functional groups at their edges, which could provide active sites to react with aqueous ammonia, as illustrated in Fig. 4a.19,32,34 The covalent modification of graphene can be achieved in four different ways: nucleophilic substitution, electrophilic addition, condensation, and addition. The main reactive sites in the nucleophilic substitution reaction are the epoxy groups of graphene oxide or graphene. The amine (–NH2) functionality of the modifiers bearing a lone pair of electrons attacks the epoxy groups of the graphene oxide or graphene. In comparison to other methods, nucleophilic substitution occurs very easily, at room temperature and in an aqueous medium. Therefore, this method has been considered to be a promising method for large-scale production of functionalized graphene.16 In general, ammonia can interact with carboxylic functional groups at the edges of the GNSs as either a Brønsted acid, to give NH4+, or a Lewis acid, to give –NH2,32 as illustrated in Fig. 4b and c, which is further supported by the FT-IR spectra of the AMGNSs in Fig. 5. After ammonia treatment, the AMGNSs have absorption peaks at wave numbers of 3430 cm−1 (the O–H stretching vibration of the terminal carboxyl groups), 1560 cm−1 (the N–H bending vibration of the primary amide), 1400 cm−1 (the C–N stretching vibration of the primary amide), and 727 cm−1 (the C–N stretching vibration of the amine). This indicates that the mixed ammonia solution successfully modified the surfaces of the GNSs, which would result in an improvement in the interfacial interactions between the AMGNSs and epoxy resin. In addition, all of the characteristic peaks exhibit a blue-shift (shift to lower wavenumbers) after ammonia modification, ascribed to the amine (–NH2) functionalization of the GNSs.3.2 Morphological analysis of AMGNS/epoxy nanocomposites
In order to evaluate the interfacial interactions between the AMGNSs and epoxy matrix and the dispersion of the AMGNSs in the samples, the freeze-fractured surfaces of the AMGNS/epoxy nanocomposites were further investigated using SEM. Fig. 6 shows that the pristine epoxy has a smooth and featureless fracture surface (Fig. 6a), and the freeze-fractured surface of the 0.1 wt% AMGNS-filled epoxy nanocomposites shows some cracks and AMGNSs can be observed to be pulled out (Fig. 6b). Then, with a further increase in AMGNS content, most of the AMGNSs disperse well and have a good crosslinking state in the epoxy resin (Fig. 6c and d). Moreover, a linear stripe morphology of the AMGNSs in epoxy resin is also observed in Fig. 6c and d. The fracture surface of neat epoxy is comparatively smooth, indicating a lower ductility. By adding AMGNSs into the epoxy resin, the toughness increased with AMGNS content, making the fracture surface much rougher. These results indicate that the AMGNSs may inhibit the propagation of cracks and thus increase the strain energy required for fracture. | ||
| Fig. 6 SEM images of the freeze-fractured surfaces of AMGNS/epoxy nanocomposites with AMGNS contents of (a) 0 wt%, (b) 0.1 wt%, (c) and (d) 0.5 wt%. | ||
3.3 Tensile properties of AMGNS/epoxy nanocomposites
The effects of AMGNS content on the tensile properties of the AMGNS/epoxy nanocomposites are shown in Fig. 7. From Fig. 7a and b, it can be seen that both the tensile strength and elongation at break of the AMGNS/epoxy nanocomposites increased steadily with increasing weight fraction of AMGNSs up to 0.5 wt%, and dropped slightly at 1 wt%. The tensile strength and elongation of the 0.5 wt% AMGNS-filled AMGNS/epoxy nanocomposites are as high as 67.28 MPa and 4.29% respectively, which are increased by 27.84% and 63.74% respectively compared with those of pristine epoxy. From Fig. 7c, it can be seen that the most significant improvement in the tensile modulus was obtained at 0.1 wt% AMGNSs, reaching 2.81 GPa, which was an increase of 14.16% compared to pristine epoxy.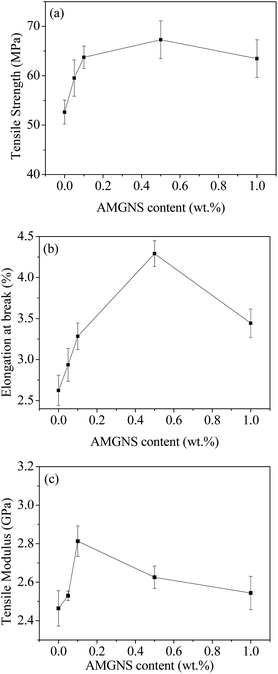 | ||
| Fig. 7 The effects of AMGNS loading content (wt%) on the tensile properties of AMGNS/epoxy nanocomposites: tensile strength (a), elongation at break (b) and tensile modulus (c). | ||
Representative tensile stress versus strain curves are shown in Fig. 8. It can be seen that both the tensile strength and the area under the stress–strain curve reach the maximum value at an AMGNS loading of 0.5 wt%. Combining Fig. 7 and 8, we can conclude that the proper surface-modification of AMGNSs can result in a significant improvement in tensile properties due to the homogeneous dispersion of AMGNSs in epoxy resin and the strong interfacial interactions between the filler and epoxy resin, resulting in effective load transfer from the epoxy resin to the AMGNS filler. However, the lower strength at higher AMGNS concentration (≥1.0 wt%) can be attributed to the inevitable aggregation of AMGNSs at high concentration.18,35
3.4 Flexural properties of AMGNS/epoxy nanocomposites
Fig. 9 shows the results of the three-point bending tests of the AMGNS/epoxy nanocomposites. It is found that all samples filled with AMGNSs show significant improvement in flexural properties compared to pristine epoxy. As can be seen, the 0.1 wt% AMGNS-filled epoxy nanocomposites shows a maximum flexural strength as high as 135.58 MPa, which is an increase of 94.38% compared to pristine epoxy and almost an increase of 37.32% compared to 0.5 wt% AMGNS-filled epoxy nanocomposites. Meanwhile, the 0.5 wt% AMGNS-filled epoxy nanocomposite shows a maximum flexural modulus as high as 3.48 GPa, which is an increase of 7.75% compared to pristine epoxy. Then, it is found that when the loading of AMGNSs reaches 1.0 wt%, the flexural properties of the AMGNS/epoxy nanocomposites begin to decline due to the agglomeration of AMGNSs at high content. The above studies agree with previous work that the addition of graphene nanoplatelets results in an improvement in the mechanical properties of nanocomposites even at a small amount of filler.18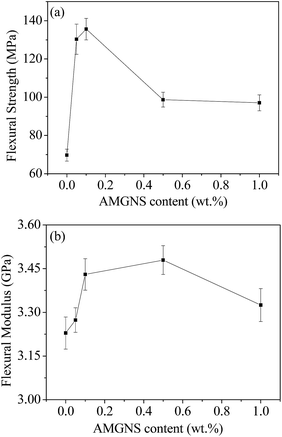 | ||
| Fig. 9 The effects of AMGNS loading content (wt%) on the flexural properties of AMGNS/epoxy nanocomposites: flexural strength (a) and flexural modulus (b). | ||
3.5 Impact strength of AMGNS/epoxy nanocomposites
The impact strengths of the AMGNS/epoxy nanocomposites are shown in Fig. 10. From Fig. 10, it can be seen that the impact strength of the AMGNS/epoxy nanocomposites improves with increasing weight fraction of AMGNSs in the epoxy matrix and increases by 34.3% from 15.65 to 21.02 MPa at an AMGNS loading of 0.1 wt%. This remarkable influence on the impact strength of nanocomposites at low AMGNS loading can be attributed to good dispersion and strong interfacial interactions, which can effectively hinder the formation and propagation of cracks in the samples.36,37 As a result, the surfaces of the nanocomposites shown in Fig. 6b are rougher than those of the pure epoxy matrix shown in Fig. 6a. Then, the impact strength of the AMGNS/epoxy nanocomposites gradually decreases with further a increase in the AMGNS content due to the weak dispersion and bad processability of the nanocomposites. The negative deteriorating effect of the AMGNS aggregation would overwhelm the toughening effect of the AMGNSs indicated by the rough fracture surfaces for the high AMGNS content of 0.5 wt% (Fig. 6d). Thus, a decrease in the composite impact strength is observed on the addition of graphene in a relatively high amount. | ||
| Fig. 10 The effects of AMGNS loading content (wt%) on the impact strength of the AMGNS/epoxy nanocomposites. | ||
3.6 DMA of AMGNS/epoxy nanocomposites
DMA provides information on the storage modulus and loss angle tangent curves of the AMGNS/epoxy nanocomposites as a function of temperature.9 As shown in Fig. 11a, the storage modulus of the AMGNS/epoxy nanocomposites improves continuously with increasing AMGNS content in the epoxy matrix. The storage modulus has obviously increased from 0.83 GPa for pristine epoxy to 1.31 GPa for the 1.0 wt% AMGNS-filled epoxy nanocomposites at the initial temperature (50 °C), which is a 57.83% increase. The storage modulus of all samples falls with temperature due to the transition from the glassy plateau to the rubbery plateau. The Tg values were taken as the maximum of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves. As shown in Fig. 11b, the glass transition temperature (Tg) began at about 133 °C for pristine epoxy. The Tg values of the AMGNS/epoxy nanocomposites containing a low content of AMGNSs (≤0.5 wt%) shift to higher temperatures of about 137–141 °C and have a narrower temperature range due to the strong interfacial interactions between the AMGNSs and epoxy matrix. From Fig. 11a and b, the mechanical improvement could be attributed to the high specific surface area of the AMGNSs with their wrinkled structure and the enhanced interfacial interactions of filler–matrix.38
δ curves. As shown in Fig. 11b, the glass transition temperature (Tg) began at about 133 °C for pristine epoxy. The Tg values of the AMGNS/epoxy nanocomposites containing a low content of AMGNSs (≤0.5 wt%) shift to higher temperatures of about 137–141 °C and have a narrower temperature range due to the strong interfacial interactions between the AMGNSs and epoxy matrix. From Fig. 11a and b, the mechanical improvement could be attributed to the high specific surface area of the AMGNSs with their wrinkled structure and the enhanced interfacial interactions of filler–matrix.38
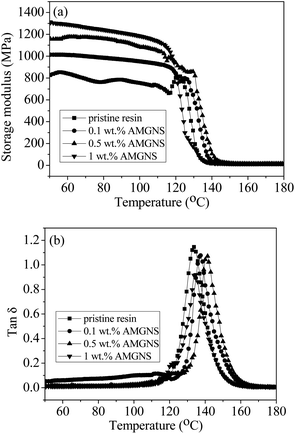 | ||
Fig. 11 The effects of AMGNS loading content (wt%) on DMA of the AMGNS/epoxy nanocomposites: (a) storage modulus spectra and (b) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ spectra. δ spectra. | ||
4. Conclusions
GNSs were amine-functionalized using NH3·H2O and H2O2 to achieve a better affinity to epoxy matrix. The presence of the functional groups was verified by FT-IR spectroscopy. The effective modification of GNSs with ammonia was confirmed to improve the interfacial interactions between the AMGNSs and epoxy resin, resulting in an improvement in mechanical properties. Thus, AMGNSs could play a reinforcing role in the epoxy matrix.The mechanical results showed that the addition of AMGNSs at 0.1 wt% loading into the pristine epoxy could achieve the most significant improvements in tensile modulus (+14.16%), flexural strength (+94.38%) and impact strength (34.3%). Moreover, the most significant improvements in tensile strength (+27.84%) and flexural modulus (+7.75%) were attained with AMGNSs at a 0.5 wt% content. The glass transition temperature (Tg) of pristine epoxy increased from 133 to 137 °C at 0.1 wt% AMGNSs and 141 °C at 0.5 wt% AMGNSs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 50672004), National High-Tech Research and Development Program (2008AA03Z513) and Doctoral Fund of Ministry of Education of China (20120010110001).Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsow, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- G. Wang, J. Yang, J. Park, X. Gou, B. Wang, H. Liu and J. Yao, J. Phys. Chem. C, 2008, 112, 8192–8195 CAS.
- G. Wang, X. Shen, J. Yao and J. Park, Carbon, 2009, 47, 2049–2053 CrossRef CAS PubMed.
- X. Li, X. Wang, L. Zhang, S. Lee and H. Dai, Science, 2008, 319, 1229–1231 CrossRef CAS PubMed.
- M. Martin-Gallegoa, R. Verdejoa, M. A. Lopez-Manchadoa and M. Sangermano, Polymer, 2011, 52, 4664–4669 CrossRef PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- I. Zaman, T. T. Phan, H. C. Kuan, Q. Meng, L. T. Bao La, L. Luong, O. Youssf and J. Ma, Polymer, 2011, 5, 1603–1611 CrossRef PubMed.
- N. Roy, R. Sengupta and A. K. Bhowmick, Prog. Polym. Sci., 2012, 37, 781–819 CrossRef CAS PubMed.
- C. Bao, Y. Guo, L. Song, Y. Kan, X. Qian and Y. Hu, J. Mater. Chem., 2011, 21, 13290–13298 RSC.
- H. Kim, Y. Miura and C. W. Macosko, Chem. Mater., 2010, 22, 3441–3450 CrossRef CAS.
- Y. Shao, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Electroanalysis, 2010, 22, 1027–1036 CrossRef CAS.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- J. R. Potts, D. R. Dreyer, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5–25 CrossRef CAS PubMed.
- T. Kuila, S. Bose, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Prog. Mater. Sci., 2012, 57, 1061–1105 CrossRef CAS PubMed.
- R. Sengupta, M. Bhattacharya, S. Bandyopadhyay and A. K. Bhowmick, Prog. Polym. Sci., 2011, 36, 638–670 CrossRef CAS PubMed.
- M. A. Rafiee, J. Rafiee, Z. Wang, H. Song, Z. Z. Yu and N. Koratkar, ACS Nano, 2009, 3, 3884–3890 CrossRef CAS PubMed.
- X. Wang, W. Xing, P. Zhang, L. Song, H. Yang and Y. Hu, Compos. Sci. Technol., 2012, 72, 737–743 CrossRef CAS PubMed.
- S. Chatterjee, J. W. Wang, W. S. Kuo, N. H. Tai, C. Salzmann, W. L. Li, R. Hollertz, F. A. Nüesch and B. T. T. Chu, Chem. Phys. Lett., 2012, 531, 6–10 CrossRef CAS PubMed.
- S. Zhao, L. S. Schadler, R. Duncan, H. Hillborg and T. Auletta, Compos. Sci. Technol., 2008, 68, 2965–2975 CrossRef CAS PubMed.
- B. B. Johnsen, A. J. Kinloch, R. D. Mohammed, A. C. Taylor and S. Sprenger, Polymer, 2007, 48, 530–541 CrossRef CAS PubMed.
- A. Yasmin and I. M. Daniel, Polymer, 2004, 45, 8211–8219 CrossRef CAS PubMed.
- P. Guo, X. Chen, X. Gao, H. Song and H. Shen, Compos. Sci. Technol., 2007, 67, 3331–3337 CrossRef CAS PubMed.
- J. N. Coleman, U. Khan and Y. K. Gun’ko, Adv. Mater., 2006, 18, 689–706 CrossRef CAS.
- H. Yang, C. Shan, F. Li, Q. Zhang, D. Han and L. Niu, J. Mater. Chem., 2009, 19, 8856–8860 RSC.
- T. Ramanathan, A. A. Abdala, S. Stankovich, D. A. Dikin, M. Herrera-Alonso, R. D. Piner, D. H. Adamson, H. C. Schniepp, X. Chen, R. S. Ruoff, S. T. Nguyen, I. A. Aksay, R. K. Prud’Homme and L. C. Brinson, Nat. Nanotechnol., 2008, 3, 327–331 CrossRef CAS PubMed.
- J. Zhu, Nat. Nanotechnol., 2008, 3, 528–529 CrossRef CAS PubMed.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350–1375 CrossRef CAS PubMed.
- K. A. Worsley, P. Ramesh, S. K. Mandal, S. Niyogi, M. E. Itkis and R. C. Haddon, Chem. Phys. Lett., 2007, 445, 51–56 CrossRef CAS PubMed.
- S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 128, 7720–7721 CrossRef CAS PubMed.
- M. Seredych and T. J. Bandosz, J. Phys. Chem. C, 2007, 111, 15596–15604 CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- P. Guo, H. Song and X. Chen, J. Mater. Chem., 2010, 20, 4867–4874 RSC.
- X. J. Shen, Y. Liu, H. M. Xiao, Q. P. Feng, Z. Z. Yu and S. Y. Fu, Compos. Sci. Technol., 2012, 72, 1581–1587 CrossRef CAS PubMed.
- J. P. Yang, G. Yang, G. Xu and S. Y. Fu, Compos. Sci. Technol., 2007, 67, 2934–2940 CrossRef CAS PubMed.
- L. Wang, K. Wang, L. Chen, Y. Zhang and C. He, Composites, Part A, 2006, 37, 1890–1896 CrossRef PubMed.
- M. A. Rafiee, J. Rafiee, Z. Z. Yu and N. Koratkar, Appl. Phys. Lett., 2009, 95, 223103 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |