Preparation of a magnetically separable CoFe2O4 supported Ag nanocatalyst and its catalytic reaction towards the decolorization of a variety of dyes†
Abstract
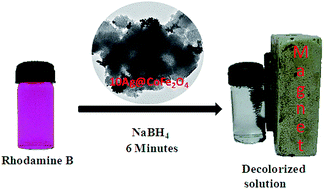
This study deals with the exploration of CoFe2O4 supported Ag nanoparticles as a catalyst for the decolorization of various dyes (such as 4-nitrophenol, Congo red, rhodamine B) and dye mixtures by employing a reduction reaction with excess NaBH4 in an aqueous medium. Nanocatalysts with 10 wt% Ag loading (10Ag@CoFe2O4) exhibited very high catalytic activity and dye solutions were found to be decolorized within 4 to 6 minutes. A simple method for the preparation of a catalyst (10Ag@CoFe2O4) was reported, which exhibited very high catalytic efficiency towards the decolorization of dyes with different chemical structures as well as demonstrating its magnetic properties for easy separation from reaction mixtures and its reusability.

 Please wait while we load your content...
Please wait while we load your content...