Development of voriconazole loaded large porous particles for inhalation delivery: effect of surface forces on aerosolisation performance, assessment of in vitro safety potential and uptake by macrophages†
Sumit Aroraa,
Rahul R. Mahajana,
Varun Kushwaha,
Dipesh Baradiab,
Ambikanandan Misrab and
Sanyog Jain*a
aCentre for Pharmaceutical Nanotechnology, Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Sector 67, S.A.S. Nagar, Mohali, Punjab-160062, India. E-mail: sanyogjain@niper.ac.in; sanyogjain@rediffmail.com; Fax: +91 172-2214692; Tel: +91 172-2292055
bTIFAC-CORE in NDDS, Pharmacy Department, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda, Kalabhavan, Vadodara-390 001, Gujarat, India
First published on 21st April 2015
Abstract
The present study investigated the development of a dry powder inhalable formulation of voriconazole (VRZ) using quality by design (QbD) principles and assessment of its suitability for administration in the lungs. VRZ loaded large porous particles (VLPP) were extensively optimised using I-optimal design investigating the four factors (polymer type, porogen concentration, drug loading and homogenisation speed) at three different levels. Formulations were evaluated for desirable critical quality attributes (CQAs) such as particle size, drug entrapment efficiency, aerodynamic performance, porosity, surface energy, in vitro drug release, macrophage uptake and safety. A design space satisfying all CQAs was identified only in the case of VLPP fabricated from poly-lactide polymer (PLA). Statistical analyses suggest that all the factors and their higher order interactions influenced the morphology and physical properties of VLPP. Optimised VLPP exhibited a VRZ loading of 4.85 ± 0.39%, porosity of 0.17 ± 0.02 and median volume diameter of 8.84 ± 0.12 μm, measured with laser diffraction. Moreover, their mass median aerodynamic diameter (2.85 ± 0.38 μm) and fine particle fraction (FPF) (27.3 ± 2.7%), as measured by an 8-stage Anderson Cascade Impactor, were suitable for pulmonary delivery. VLPP was found to sustain the release of VRZ for over 7 days. Increase in the surface energy of VLPP promoted enhanced aerosolisation. No cytotoxic and inflammatory (IL-8) effect was observed when A549 cells were incubated with VLPP. In addition, VLPP was large enough to evade macrophage uptake, thus prolonging the residence time of VLPP at the site of action. Overall, this study suggests the suitability of VLPP for targeting invasive pulmonary aspergillosis by inhalation.
1. Introduction
With the increase in the complexity of modern lifestyles, there has been a significant rise in the patient population undergoing modern medical interventions and those suffering from immunosuppressive diseases such as AIDS.1–3 Fungus, an opportunistic organism, is known to cause invasive infections in immunocompromised patients resulting in high morbidity as well as mortality. Despite such increases in incidences of invasive pulmonary fungal infections in immunocompromised patient populations, these infections are still underdiagnosed and a targeted approach to treat these infections is relatively underexplored compared with other infectious diseases.4,5 Considering the global scenario, the patient population suffering from life-threatening fungal infections is growing at an alarming rate and finding treatment modalities to combat the menace of such infections has become a much higher priority.4,6Pulmonary delivery of antifungals is increasingly gaining attention since the major route of exposure of pathogenic fungal spores is through inhalation, which is often the first step in the pathogenesis of invasive fungal infections in the lungs.7 VRZ, a second-generation triazole antifungal agent, represents the primary salvage therapy for the treatment of invasive pulmonary aspergillosis (IPA), given either through oral or intravenous route.3,7 However, its use is sometimes limited by excessive side effects and significant drug–drug interactions due to cytochrome P450 inhibitory potential of VRZ. Targeting of airway to deliver antifungal drugs represent a viable and attractive solution to overcome this problem.7 A number of reports on inhaled antifungals, particularly amphotericin B and itraconazole have demonstrated favourable lung retention profile, improved outcomes in animal models and probable clinical utility.8–14 However, pulmonary delivery of VRZ has received comparatively little attention. Tolman et al. evaluated single and multiple dose pharmacokinetics of inhaled VRZ solution in mice and demonstrated attainment of clinically relevant therapeutic concentration.15 In another work done by the same group, particulate formulation of VRZ prepared by thin film freezing were administered to mice and founded to attain high concentrations in the lungs as well as in plasma.16,17 However, both the studies showed rapid clearance of VRZ from the lungs, thereby limiting its residence time.
Sinha et al. evaluated VRZ loaded poly-lactide-co-glycolide nanoparticles for delivery in the lungs. However, the formulation was optimised using conventional (one variable at a time) approach and does not take into account effect of all factors (formulation and process factors) at once on the desirable CQAs.18
Large porous particles fabricated from biodegradable polymers represent a suitable delivery vehicle capable of delivering drugs to the middle and lower regions of the lungs.19,20 In addition, they also offer the advantage of escaping macrophage uptake by virtue of their large geometric size and sustaining the release of drug, thereby prolonging the residence time of encapsulated drug in the lungs.21–23 The present investigation utilizes the QbD principles to develop a controlled release large porous microparticulate formulation fabricated from biodegradable polymers for the pulmonary delivery of VRZ. To best of our knowledge and thorough literature survey, this is the first report investigating in detail the development and characterisation of VLPP for the targeted treatment of IPA based on QbD principles. VLPP was extensive characterised in terms of physicochemical characteristics, entrapment efficiency, aerosolisation potential, surface energy, in vitro release of VRZ, macrophage uptake and in vitro safety studies to determine suitability of VLPP for lung delivery.
2. Materials and methods
2.1 Materials
Poly(D,L-lactide-co-glycolide) (PLGA) polymers, Resomer® RG502 (lactide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycolide = 50
glycolide = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), Resomer® RG752H (lactide
50), Resomer® RG752H (lactide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycolide = 75
glycolide = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) and poly(D,L lactide) (PLA, molecular weight 10
25) and poly(D,L lactide) (PLA, molecular weight 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–18
000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, viscosity 0.16–0.24) were purchased from Boehringer Ingelheim (Ingelheim, Germany). Poly(vinyl alcohol) (PVA) with average MW 30–70 kDa and ammonium bicarbonate were purchased from Sigma (St. Louis, MO, USA). VRZ (Pharma Grade) was a kind gift from Ranbaxy Laboratories (Gurgaon, India). All other chemicals and solvents were of the highest analytical grade commercially available.
000, viscosity 0.16–0.24) were purchased from Boehringer Ingelheim (Ingelheim, Germany). Poly(vinyl alcohol) (PVA) with average MW 30–70 kDa and ammonium bicarbonate were purchased from Sigma (St. Louis, MO, USA). VRZ (Pharma Grade) was a kind gift from Ranbaxy Laboratories (Gurgaon, India). All other chemicals and solvents were of the highest analytical grade commercially available.
2.2 Preparation of VLPP
VLPP were prepared using a water-in-oil-in-water (w/o/w) double emulsion method.22 Experiments were performed as per experiment design created using statistical software SAS JMP® 10 (SAS Institute Inc., Cary, USA). Briefly, PLGA or PLA and VRZ were dissolved in 3.75 mL ethyl acetate. Freshly-prepared ammonium bicarbonate solution was added to the polymer solution (w/w percent with respect to polymer) and the mixture was sonicated in ice bath using a probe sonicator (Misonix, USA) for 30 s alternating on and off (1 min total sonication time) at 25% amplitude. The sonicated mixture was added to 25 mL of 1% polyvinyl alcohol (PVA) solution while it was being homogenized at a rate of 4000–8000 rpm using a Polytron PT 4000 (Switzerland). It was then poured into 50 mL of water, and stirred for 8 h at room temperature to remove ethyl acetate. The particles were collected by centrifugation at 4000 rpm for 5 min and washed 3 times with distilled water. The suspension was then lyophilised to obtain the dry powder.2.3 Experimental design and protocol
A I-optimal experimental design was custom built using SAS JMP® 10 (SAS Institute Inc., Cary, USA) in order to investigate the effect of various factors, viz. polymer type, theoretical drug loading, homogenization speed and porogen concentration (% w/w with respect to the polymer) on the formation of VLPP. Preliminary experiments (data not shown) helped in the selection of factors which were explored at three levels (as broad as possible) as mentioned in Table 1. The selection of experimental design was based on Fraction of Design Space (FDS) plot such that 90% of the experimental domain was covered with minimum variance. The software yielded a total of 24 experiments with 2 centre points to explore the experimental domain. Experiments were run in random order to increase the predictability of the model. The experimental design and the conditions are mentioned in Table 2.| Factor | Nature of factor | Levels | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Polymer type | Categorical | PLGA 502 | PLGA 752H | PLA |
| Drug loading (%) | Numeric | 5 | 10 | 15 |
| Homogenisation speed (rpm) | Numeric | 4000 | 6000 | 8000 |
| Porogen concentration (% w/w) | Numeric | 0 | 3.75 | 7.5 |
| Run order | Polymer type | Drug loading (%) | Homogenisation speed (rpm) | Porogen (% w/w) |
|---|---|---|---|---|
| 1 | PLA | 10 | 8000 | 7.5 |
| 2 | PLGA 752H | 10 | 4000 | 7.5 |
| 3 | PLGA 502 | 15 | 6620 | 3.75 |
| 4 | PLGA 502 | 15 | 4000 | 7.5 |
| 5 | PLGA 502 | 10 | 8000 | 7.5 |
| 6 | PLGA 752H | 5 | 6000 | 0 |
| 7 | PLGA 752H | 15 | 6000 | 7.5 |
| 8 | PLGA 502 | 5 | 8000 | 0 |
| 9 | PLA | 15 | 8000 | 0 |
| 10 | PLA | 10 | 4000 | 0 |
| 11 | PLGA 502 | 5 | 6620 | 7.5 |
| 12 | PLA | 15 | 4000 | 7.5 |
| 13 | PLGA 752H | 10 | 8000 | 0 |
| 14 | PLA | 10 | 6000 | 3.75 |
| 15 | PLGA 752H | 5 | 8000 | 3.75 |
| 16 | PLGA 752H | 15 | 4000 | 3.75 |
| 17 | PLGA 752H | 5 | 6000 | 3.75 |
| 18 | PLA | 5 | 8000 | 7.5 |
| 19 | PLGA 752H | 10 | 6000 | 2.5 |
| 20 | PLGA 502 | 5 | 4000 | 0 |
| 21 | PLGA 502 | 10 | 6000 | 7.5 |
| 22 | PLGA 502 | 15 | 4000 | 3.75 |
| 23 | PLA | 10 | 4000 | 0 |
| 24 | PLA | 5 | 8000 | 3.75 |
Prediction model was estimated using the least squares method and is given according to the following eqn (1):
| Y = βo + ∑βiXi + ∑βii(Xi − μi)2 + ∑βij(Xi − μi)(Xj − μj) + ∈ | (1) |
Main effects, first order and second order interactions were taken into consideration for the construction of prediction model. Model was accepted if it exhibited no lack of fit, no correlation in the residual plots and the residuals were normally distributed. Model was validated by selecting three check points in the design space. These points correspond to the spots in the optimality region where the model predicts desirable VLPP CQAs.
2.4 Solid state characterisation
2.5 Response measurements
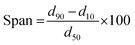 | (2) |
tMMAD for spherical particles was calculated by taking into consideration the geometric diameter of VLPP and the tapped density according to the eqn (3):
 | (3) |
The morphology of the optimised VLPP (model validation batches) was further analysed using conventional tapping mode, atomic force microscopy (AFM) (Nanoscope IIIa controller with Multimode AFM, Veeco, USA) and compared with the non-porous VRZ loaded microsphere formulation. Samples were mounted on carbon sticky tabs and imaged in air using tapping mode tips (OTESPA, Veeco, USA) at a scan rate of 0.5 Hz. Pore depth was calculated using section analysis. The surface roughness was quantified by post image analysis. Individual particles (n ≥ 5) were analysed over an area of 1 μm × 1 μm areas and root mean square roughness was calculated using eqn (4):
 | (4) |
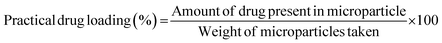 | (5) |
 | (6) |
An accurately weighed quantity of the optimised VLPP (5 mg) was placed in the dialysis membrane (12 kD) (Sigma Aldrich, USA) and suspended in 20 mL of the dissolution medium, phosphate buffer saline (pH 7.4, 10 mM) containing 0.1% Tween 80 in order to maintain sink conditions. At fixed intervals of time, samples (400 μL) were withdrawn and replenished with freshly prepared buffer. The samples were then analysed for the VRZ content using validated HPLC. All the experiments were performed at 37 ± 0.5 °C and 100 rpm. The cumulative percentage VRZ released was calculated, and the mean values and standard deviations were reported. The release profile of VRZ from VLPPs were further mathematically modeled to determine the release kinetics of VRZ from VLPPs.
2.6 Cell culture
Lung adenocarcinoma (A549) and murine macrophage cell line (RAW 264.7) were procured from National Centre for Cell Science (NCCS), Pune, India. A549 cells were cultured under conditions as previously described by Sanyog Jain et al.25 RAW 264.7 were cultured using the air-liquid (gaseous phase) culture methods as described by Kim et al.26 and Hwang et al.27 with minor modifications. The macrophages obtained by these methods are expected to possess phagocytosis property similar to the primary alveolar macrophages Φ.27,28 Briefly, cells were suspended at a concentration of 2–3 × 10−6 cells per mL in DMEM containing 10% (w/v) FBS and antibiotics. An aliquot (1 mL) of the cell suspension was seeded on a cell culture insert (Transwell®, 0.4 μm pore size, 0.33 cm2 surface area, Corning Costar, Lowell, MA, USA), and 3 mL of medium was added to each of the 6 wells (Costars, Corning 261 Inc., NY, USA). The plates were then incubated at 37 °C in a 5% CO2 atmosphere. After a 12 h incubation, the medium in the culture insert was removed and the cells was gently washed with PBS (200 μL) two times. Fresh DMEM containing 10% FBS was added to each well.2.7 Principal component analysis and other statistical analysis
Principal Component Analysis (PCA) computed with SAS JMP® 10 was used to obtain visual overview of interactions between factors and responses. PCA reduces the complexity of the data set by introducing principal components. The first principal component (PC1) accounts for the largest variation across the experimental domain while the second principal component (PC2) orthogonal to the first component accounts for the second largest variation. Loading plot and score plot were plotted to gain useful insights in the complex data set obtained from the DoE runs. Correlation matrix was also computed in order to observe dependency of various factors and responses among one another.29,30All other statistical calculations were performed with GraphPad Instat version 5.1 (GraphPad Software, Inc., San Diego California). The data were analysed either by one-way analysis of variance (between several objects) or by unpaired t-test (between two objects) to determine significant differences. Statistical significance was based on a probability value of less than 0.05 and in some cases 0.01.
3. Results
VLPP made from PLA exhibited desirable particle characteristics for deep lung targeting. Results of the extensive particle characterisation are mentioned in Table 3. In all the 24 experiments, white powder material was obtained. The yield was above 70% in all the experimental runs.| DoE run | AD (μm) | FPF (%) | GSD | SPAN | tMMAD (μm) | Surface energy (mJ m−2) | Geometric diameter (μm) | Practical loading (%) | Entrapment efficiency (%) | Poured density (g cm−3) | Tapped density (g cm−3) | Porosity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a AD – aerodynamic diameter, FPF – fine particle fraction, GSD – geometric standard deviation, tMMAD – theoretical mass median aerodynamic diameter. Values in parenthesis represent S.D. (n = 6) except in case of AD, FPF and GSD where n = 3. | ||||||||||||
| 1 | 4.82 (0.89) | 13.25 (2.34) | 2.74 (0.65) | 1.60 (0.32) | 2.62 (0.11) | 44.76 (1.98) | 6.80 (0.67) | 6.00 (0.74) | 60.00 (7.4) | 0.109 (0.018) | 0.149 (0.012) | 0.272 (0.062) |
| 2 | 3.28 (0.54) | 25.99 (4.55) | 3.23 (0.98) | 7.78 (1.49) | 7.36 (1.16) | 53.23 (1.45) | 46.00 (3.34) | 3.10 (0.39) | 31.00 (3.90) | 0.016 (0.005) | 0.026 (0.008) | 0.385 (0.003) |
| 3 | 3.94 (0.62) | 24.04 (3.56) | 3.82 (0.54) | 1.30 (0.23) | 2.43 (0.08) | 54.24 (1.23) | 10.10 (0.89) | 3.20 (0.20) | 21.33 (1.33) | 0.047 (0.008) | 0.058 (0.004) | 0.193 (0.083) |
| 4 | 11.06 (1.78) | 19.89 (2.87) | 8.10 (1.78) | 6.04 (0.56) | 8.69 (0.55) | 49.76 (2.78) | 17.62 (1.56) | 3.40 (0.87) | 22.67 (5.80) | 0.162 (0.034) | 0.244 (0.031) | 0.341 (0.056) |
| 5 | 4.03 (0.32) | 22.00 (4.34) | 5.12 (0.76) | 17.67 (3.23) | 4.25 (0.25) | 51.40 (1.56) | 11.25 (1.23) | 3.05 (0.65) | 30.50 (6.54) | 0.104 (0.009) | 0.143 (0.017) | 0.271 (0.024) |
| 6 | 14.01 (1.56) | 21.67 (1.98) | 6.94 (1.09) | 55.26 (6.34) | 15.88 (1.07) | 54.36 (2.34) | 24.00 (2.76) | 1.95 (0.16) | 39.00 (3.24) | 0.362 (0.037) | 0.439 (0.059) | 0.173 (0.027) |
| 7 | 5.49 (0.78) | 27.75 (3.78) | 3.58 (0.82) | 2.54 (0.32) | 6.28 (0.38) | 59.87 (1.56) | 28.14 (2.89) | 4.90 (0.87) | 32.67 (5.87) | 0.028 (0.006) | 0.050 (0.006) | 0.444 (0.054) |
| 8 | 4.26 (0.23) | 23.43 (1.34) | 3.82 (0.67) | 1.88 (0.27) | 4.77 (0.24) | 52.58 (1.76) | 7.80 (0.78) | 1.68 (0.12) | 33.60 (2.43) | 0.298 (0.049) | 0.375 (0.038) | 0.209 (0.051) |
| 9 | 2.67 (0.12) | 19.48 (2.78) | 2.45 (0.47) | 1.24 (0.33) | 1.62 (0.19) | 54.65 (1.67) | 3.00 (0.12) | 6.13 (0.76) | 40.87 (5.07) | 0.248 (0.075) | 0.294 (0.067) | 0.167 (0.067) |
| 10 | 8.92 (0.76) | 14.65 (1.05) | 4.70 (0.54) | 1.32 (0.19) | 9.70 (0.26) | 47.31 (2.67) | 16.29 (1.45) | 5.12 (0.63) | 51.20 (6.32) | 0.326 (0.034) | 0.355 (0.019) | 0.083 (0.047) |
| 11 | 7.38 (0.45) | 20.71 (3.67) | 7.26 (0.73) | 6.04 (1.33) | 5.67 (0.19) | 49.94 (1.67) | 15.62 (0.56) | 2.42 (0.14) | 48.40 (2.80) | 0.091 (0.006) | 0.132 (0.009) | 0.311 (0.002) |
| 12 | 5.39 (0.14) | 26.20 (2.34) | 3.61 (0.77) | 8.83 (0.10) | 7.75 (0.57) | 52.87 (1.38) | 42.08 (3.24) | 5.06 (0.56) | 33.73 (3.73) | 0.022 (0.005) | 0.034 (0.005) | 0.358 (0.053) |
| 13 | 6.57 (0.34) | 12.85 (1.98) | 3.93 (0.63) | 10.95 (1.23) | 2.40 (0.09) | 42.08 (0.96) | 3.75 (0.23) | 3.94 (0.45) | 39.40 (4.50) | 0.384 (0.041) | 0.409 (0.029) | 0.063 (0.034) |
| 14 | 3.44 (0.14) | 25.21 (3.56) | 3.44 (0.34) | 3.01 (0.65) | 3.81 (0.24) | 57.97 (0.67) | 6.84 (0.42) | 4.50 (0.29) | 45.00 (2.90) | 0.262 (0.056) | 0.310 (0.039) | 0.161 (0.076) |
| 15 | 16.31 (1.97) | 20.04 (1.67) | 8.46 (2.23) | 3.53 (0.23) | 16.75 (0.58) | 48.23 (0.78) | 33.95 (2.45) | 2.56 (0.34) | 51.20 (6.8) | 0.181 (0.016) | 0.244 (0.017) | 0.259 (0.014) |
| 16 | 11.34 (0.89) | 18.46 (2.67) | 5.16 (1.07) | 3.47 (0.45) | 10.61 (0.29) | 49.34 (1.89) | 32.00 (3.23) | 2.82 (0.19) | 18.80 (1.27) | 0.084 (0.010) | 0.110 (0.006) | 0.238 (0.049) |
| 17 | 7.24 (0.93) | 17.70 (1.89) | 3.38 (0.45) | 3.91 (0.48) | 6.84 (0.26) | 50.35 (1.65) | 16.45 (1.67) | 3.45 (0.29) | 69.00 (5.8) | 0.138 (0.013) | 0.173 (0.013) | 0.203 (0.015) |
| 18 | 3.24 (0.16) | 24.39 (2.85) | 2.19 (0.65) | 16.80 (2.98) | 3.74 (0.06) | 58.71 (1.76) | 12.61 (0.87) | 4.20 (0.56) | 84.00 (11.2) | 0.061 (0.008) | 0.088 (0.003) | 0.308 (0.067) |
| 19 | 18.74 (2.41) | 11.45 (1.23) | 5.16 (1.23) | 35.25 (3.98) | 24.61 (1.56) | 40.53 (0.64) | 40.41 (3.87) | 4.30 (0.79) | 43.00 (7.91) | 0.327 (0.039) | 0.372 (0.047) | 0.120 (0.006) |
| 20 | 14.35 (1.67) | 8.05 (1.34) | 7.10 (2.34) | 2.77 (0.17) | 9.49 (0.13) | 40.76 (0.96) | 14.46 (1.82) | 2.24 (0.29) | 44.80 (5.82) | 0.399 (0.013) | 0.431 (0.012) | 0.074 (0.004) |
| 21 | 9.67 (0.45) | 16.45 (1.67) | 9.07 (1.67) | 57.72 (5.98) | 9.40 (0.59) | 50.91 (2.34) | 24.90 (2.03) | 1.38 (0.45) | 13.80 (4.50) | 0.097 (0.03) | 0.143 (0.018) | 0.332 (0.127) |
| 22 | 12.25 (1.34) | 15.52 (2.44) | 10.84 (1.98) | 2.89 (0.46) | 9.74 (0.76) | 46.80 (1.76) | 25.99 (2.45) | 1.41 (0.34) | 9.40 (2.27) | 0.117 (0.016) | 0.141 (0.022) | 0.169 (0.016) |
| 23 | 7.86 (0.87) | 18.94 (1.34) | 7.63 (0.93) | 1.65 (0.21) | 7.25 (0.34) | 49.64 (1.67) | 11.22 (1.13) | 4.70 (0.76) | 47.00 (7.60) | 0.406 (0.043) | 0.418 (0.039) | 0.029 (0.012) |
| 24 | 4.21 (0.23) | 29.48 (1.23) | 3.86 (0.29) | 2.24 (0.67) | 2.67 (0.09) | 57.51 (1.73) | 4.86 (0.19) | 3.80 (0.19) | 76.00 (3.80) | 0.247 (0.024) | 0.303 (0.021) | 0.186 (0.023) |
3.1 Design of experiments
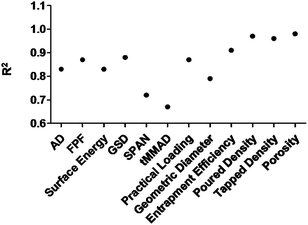
The prime most aim was to identify the design space within the experimental domain where all the CQAs were satisfied and the best settings could be determined to obtain the quality target product profile (QTPP). The desired CQAs were: (a) AD (between 1–5 μm) (b) FPF (>25%) (c) GSD (<5) (d) Geometric diameter (between 8–20 μm) (e) span (<5) (f) tMMAD (between 1–5 μm) (g) practical drug loading (>4%) (h) entrapment efficiency (>60%). The values of surface energy, poured density and tapped density were also determined and modelled. However, no limits were set for these factors. Keeping in considerations all these desirable attributes, the experimental domain was investigated. Interestingly, design space (depicted by white region in the Fig. 1) was obtained only in the case of PLA under the given set of experimental setting. VLPP prepared from PLA were only able to satisfy the criteria defined for the CQAs. R2 values varying from 0.67 to 0.98 were obtained for response variables investigated following mathematical modelling (Fig. 2) (surface profilers explaining the relationship between factors and responses measurements of VLPP fabricated from PLA has been mentioned in ESI† since design space was only identified in VLPP fabricated from PLA).
| Model validation batch | Polymer type | Drug loading | Homogenization speed | Porogen | Actual by predicted | AD (μm) | FPF (%) | Surface energy (mJ m−2) | GSD | SPAN | tMMAD (μm) | Practical loading (%) | Geometric diameter (μm) | Entrapment efficiency (%) | Poured density (g cm−3) | Tapped density (g cm−3) | Porosity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Each data value in ‘actual’ row represents an average reading of three independent batches. AD – aerodynamic diameter, FPF – fine particle fraction, GSD – geometric standard deviation, tMMAD – theoretical mass median aerodynamic diameter. | |||||||||||||||||
| I | PLA | 8 | 5400 | 3 | Predicted | 3.11 | 25.78 | 55.49 | 2.64 | 2.24 | 3.39 | 4.81 | 8.43 | 61.98 | 0.285 | 0.317 | 0.101 |
| Actual | 3.38 | 26.32 | 57.98 | 2.85 | 2.67 | 4.49 | 5.06 | 8.03 | 63.3 | 0.281 | 0.314 | 0.105 | |||||
| Prediction error (%) | 8.68 | 2.09 | 4.49 | 7.96 | 19.19 | 32.45 | 5.19 | 4.78 | 2.13 | 1.40 | 1.06 | 3.96 | |||||
| II | PLA | 7.5 | 5900 | 4 | Predicted | 2.34 | 26.59 | 56.48 | 2.11 | 4.01 | 3.26 | 4.62 | 8.17 | 65.59 | 0.235 | 0.281 | 0.164 |
| Actual | 2.56 | 27.3 | 59.87 | 1.98 | 4.47 | 4.73 | 4.85 | 8.84 | 64.78 | 0.238 | 0.287 | 0.170 | |||||
| Prediction error (%) | 9.41 | 2.63 | 6.01 | 6.16 | 11.47 | 45.09 | 4.98 | 8.02 | 1.23 | 1.28 | 2.14 | 3.53 | |||||
| III | PLA | 8.3 | 5600 | 5 | Predicted | 1.78 | 25.4 | 54.78 | 1.73 | 3.12 | 4.67 | 4.77 | 12.7 | 62.87 | 0.198 | 0.267 | 0.258 |
| Actual | 1.89 | 25.9 | 56.34 | 1.82 | 3.64 | 5.59 | 4.95 | 10.68 | 62.16 | 0.203 | 0.274 | 0.259 | |||||
| Prediction error (%) | 6.17 | 1.9 | 2.84 | 5.2 | 14.28 | 19.70 | 3.77 | 15.91 | 1.12 | 2.53 | 2.62 | 0.04 | |||||
3.2 Solid state characterisation of VLPP
XRD diffractogram of the VLPP fabricated from PLGA 502, PLGA 752H and PLA (Fig. 3A) showed absence of characteristic peaks of VRZ which was observed in the XRD spectra of raw VRZ. In addition, DSC (Fig. 3B) showed the absence of endotherm at 131 °C corresponding to the melting point of VRZ in all the microparticulate formulations. | ||
| Fig. 3 Solid state characterisation of VLPP fabricated from different polymer investigated (A) PXRD and (B) DSC analysis. | ||
3.3 Principal component analysis (PCA)
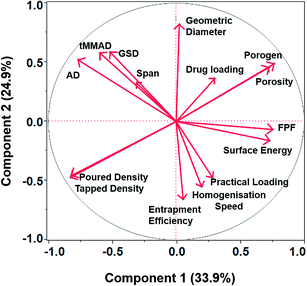
PCA is a useful statistical technique for visualizing the interactions between the factors and responses. PCA of the DoE runs helped in gaining useful insights and deriving possible correlation among various factor and responses by reducing the complexity of the data set. The principal component 1 (PC1) represented the largest variation of 33.9% which was due to homogenization speed (Fig. 4A) while principle component 2 (PC2) explained variation due to porogen concentration and accounted for 24.9% of the total variance of the data set (Fig. 4B). The third principal component accounted for 12.5% variation due to the drug loading (data not shown). All the three principal components together explain 69.6% of the total variation in the data set.Loading plot provides more visually pleasing representation of correlation between the various response variables and process parameters. Parameters and variables grouped close to each other are positively correlated while those opposite to each other are negatively related. Parameters and variables which are perpendicular to each other does not have any influence on each other. This represented a useful way to discern relationships in the complex data generated from the DoE runs (Fig. 5).
3.4 Effect of factors on response variables
SEM images further revealed that the particle morphology and surface texture were greatly influenced by the porogen concentration and polymer type. In all the cases, as the concentration of ammonium bicarbonate was increased, both the pore size and pore density were found to be increased. These results were consistent with the previous published studied utilizing this porogen.31–33 The microspheres were non-porous with 0% porogen concentration while showed highly porous structure with 7.5% porogen concentration. VLPP fabricated from PLGA 752H showed very rough surface texture at 7.5% concentration of ammonium bicarbonate.
The inherent strength of the polymeric backbone played an important role in maintaining integrity of porous microspheres. Microspheres with well-defined morphology were obtained in case of PLA at all concentrations of porogen tested while in case of PLGA 502, broken microspheres were observed at high concentration of porogen.
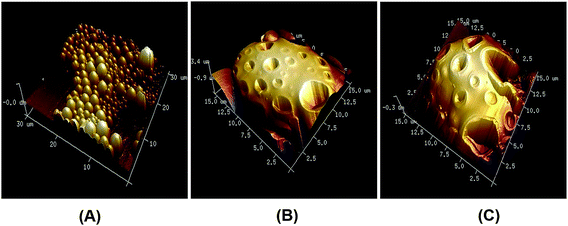
AFM topographical imaging of optimised VLPP (model validation batches) further helped in gaining useful information regarding the morphology of the prepared VLPP (Fig. 7). The nanoscopic surface characteristics were further studied, pore size and surface roughness as assessed by post image analysis are reported in Table 5.
| Porous microsphere | Pore size (μm) | Surface roughness (nm) |
|---|---|---|
| a Each data is represented as mean ± S.D. (n = 6) (** p < 0.05, *** p < 0.001). VLPP0 – VLPP fabricated with zero porogen concentration (w/w with respect to polymer) VLPP3 – VLPP fabricated with 3% porogen concentration (w/w with respect to polymer) VLPP5 – VLPP fabricated with 5% porogen concentration (w/w with respect to polymer). | ||
| VLPP0 | — | 33.22 ± 3.79 |
| VLPP3 | 0.83 ± 0.24 | 134.18 ± 37.79*** |
| VLPP5 | 1.82 ± 0.92** | 349.60 ± 38.89*** |
Homogenization speed and porogen concentration were the most important factors affecting the geometric particle size and span of VLPP as analysed by Pareto charts (data shown in ESI†). An increase in homogenization speed resulted in decrease in particle size of VLPP with narrower span index whereas porogen concentration exerted the opposite effect. Increase in porogen concentration resulted in VLPP with large geometric diameter and wider span (Table 3). These results are along the expected lines as documented in literature also.32,33
FPF of the VLPP was found to be mainly affected by the homogenization speed and higher order interactions between homogenization speed and porogen (Pareto chart analysis shown in ESI†). VLPP formulated from PLA were found to show greater FPF (>25% of their dose) as compared to VLPP fabricated from PLGA 502 or PLGA 752H. Homogenization speed and polymer type and their higher order interactions were found to influence GSD of the VLPP. High homogenization speed led to the narrow GSD of the VLPP (Table 3).
3.5 In vitro VRZ release from optimised VLPP batches
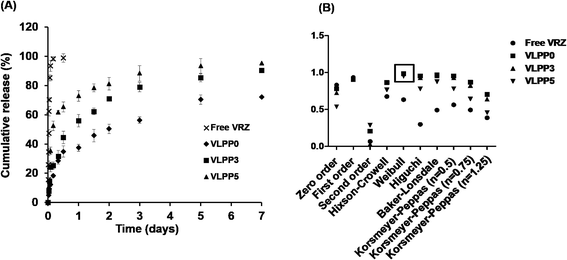
Fig. 9A demonstrate in vitro release profile of VRZ from VLPP fabricated from PLA at different porogen concentration and compared with the dissolution of free VRZ. VRZ was found to be completely released from the raw drug within 2 h and followed first order release kinetic. However, sustained release of VRZ from the VLPP was observed for a period of 7 days. VLPP prepared from 3% and 5% porogen concentration (w/w with respect to the polymer concentration) released 90.34% and 95.34% of the total VRZ loaded respectively in 7 days. Non-porous microspheres, on the other hand, released only 72.23% of the encapsulated VRZ over a period of 7 days. VRZ was released at faster rate from porous microspheres compared to the non-porous microspheres. A biphasic release profile of VRZ was observed from all the formulations. The release of VRZ from the optimised VLPP was found to follow weibull release kinetics (Fig. 9B).3.6 Safety studies
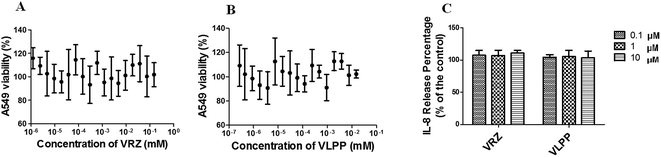
Dose response cytotoxicity profile of VRZ (1.2 nM to 150 μM) and VLPP (1.2 nM to 15 μM) were investigated to establish initial safety profile (Fig. 10A and B). The viability of cells was calculated with reference to the untreated cells where average absorbance was normalised to 100% viability. Cell viability results showed both VRZ and VLPP did not compromise cell viability and more than 90% of cells were viable following treatment with the highest concentration tested (100 μM for VRZ and 10 μM for VLPP, respectively).Basolateral media collected after 4 h exposure to the VRZ and VLPP were analysed for the inflammatory marker, IL-8 (Fig. 10C). No significant increase in the level of secretion was observed (p > 0.05).
3.7 Phagocytic uptake of microparticles
Fraction of formulations collected from ACI stages 3 through 6 correspond to particles which may deposit from trachea to alveoli. As expected, the highly porous large particles were not taken up by macrophages in 3 h even though macrophages were found to be adherent to these particles (Fig. 11A). Following washing with HBSS (5×), most of the VLPP were removed as indicated by loss of red florescence. Further, line series analysis of fluorescence along the white line (Fig. 11B–d and e) demonstrated non-overlapping of green and red fluorescence, leading to the conclusion that macrophages were not able to engulf the highly porous particles. However, it is worthy to note that some florescent signals were still seen in the macrophages even after washing with HBSS (Fig. 11B).On the other hand, most of non-porous particles were engulfed by the macrophages in 3 h (Fig. 11C and D). Following washing with HBSS (5×), small proportion of large non-porous particles not engulfed by macrophages (Fig. 11C) were removed and majority of non-porous particles were retained within the macrophages (Fig. 11D). In addition, line series analysis of fluorescence along the white line (Fig. 11D–d and e) demonstrated co-localisation of green and red fluorescence which suggested macrophages were able to engulf the non-porous particles.
4. Discussion
Sustained local effect of the drug in the lungs is mainly limited by the rapid absorption in the systemic circulation as well as phagocytic clearance of the drug delivery vehicle.23 In order to achieve sustained delivery of VRZ in the lungs, highly porous microparticles of biodegradable polymers loaded with VRZ were formulated and extensively characterised for their biopharmaceutical characteristics desirable for efficient lung delivery.QbD, in association with PCA, provided significant insights into the role played by various factors and their higher order interactions in determining the desirable CQAs of VLPP. I-optimal design was chosen since it allows the evaluation of categorical as well as numeric factors simultaneously while retaining the orthogonality of the design.34 It is important to fit the design to the experiment and not the experiment to the design. I-optimal designs also offer advantage over D-optimal designs in terms of better predictability of response parameters.34 PCA indicated that homogenization speed (PC1) and porogen concentration (PC2) were the most influential factors affecting the VLPP characteristics and must be closely monitored. Variables such as geometric diameter, span, AD and GSD of VLPP were found to be strongly dependent on the homogenization speed as indicated by high numerical score on the component 1 axis and were negatively correlated to the homogenization speed (Fig. 4 and 5). High homogenization speed led to the breakage of double emulsion into globules of smaller size resulted in VLPP with small particle size and narrow size distribution. Two-way interaction of homogenization speed and porogen concentration also played a role in determining the effective particle size of VLPP. Increase in homogenization speed led to decrease particle size of VLPP while increase in porogen concentration resulted in VLPP with larger geometric diameter as also evident from other studies.35 Thus, the effective particle size of VLPP was mainly determined by the combination of porogen concentration and homogenization speed.
Efficient delivery of drug to the alveoli can be obtained by designing inhaled formulations with aerodynamic diameter between 1–5 μm.36 It is further well documented that reduction in particle density led to decrease in aerodynamic diameter (as given by eqn (3)), while still retaining the large geometric diameter.23 Addition of ammonium bicarbonate as effervescent porogen in the polymer phase as internal aqueous core was an effective way of forming highly porous large microparticles.22 As expected, increase in the porogen concentration resulted in VLPP with increased pore size and pore density demonstrated by morphological examination by SEM (Fig. 6). In case of PLGA 502, broken VLPP were observed at high concentration of porogen. This may be because of less inherent strength of PLGA 502 which was unable to maintain integrity of the particles owing to highly porous structures. AFM imaging of the DoE model validation batches further revealed important nanoscopic characteristics of the optimised VLPP (Fig. 7). Post image analysis of AFM data revealed increase in surface roughness in case of porous particles as compared to non-porous particles (Table 5). This may be primarily because of the change in morphology of VLPP as a result of effervescence produced by ammonium bicarbonate during solidification process. Increased pore density with increased porogen concentration also resulted in concomitant decrease in the density and hence, increased porosity of the VLPP (Table 3).
Solid state characterisation of the VLPP demonstrated the absence of any crystalline VRZ within the VLPP as indicated by absence of melting endotherm and diffraction peaks of VRZ in the DSC thermogram and XRD spectra respectively (Fig. 3). Presence of amorphous form of VRZ is the indication of its stabilization in the presence of PLGA/PLA.
Complex relationships were found between the formulation and process factors and the in vitro aerosolisation performance of VLPP. This is evident by the DoE results (Table 3) as no single factor found to have significant influence on aerodynamic characteristics of VLPP. Complex interplay of process and formulation factors such as two way interactions, homogenization speed/porogen concentration and polymer type/porogen concentration influenced the aerodynamic characteristics of VLPP.
It was envisaged that the knowledge of work of cohesion and surface energetics operating within VLPP would be useful in fundamental understanding of the process of VLPP aerosolisation and dispersibility. Contact angle is a useful indicator of wettability, providing valuable information about surface energy using Lifshitz–van der Waals/acid–base approach.22 Sessile drop contact angle method is the commonly applied methodology to study the surface of compact discs. However, numerous reports have been published which revealed that compaction leads to alteration of surface morphology and hence surface free energy of powders.37 In the present study, the contact angle was determined by adhering powder to the inert support, thus allowing evaluation of surface characteristics of the powders as such without alteration. Particulate interactions at the surface include electrostatic, capillary forces and van der Waals (proximity) forces as well as mechanical interlocking.24 Complexities are further increased by presence of amorphous domains on the particle surface, impurities present and specific polar/non-polar region (dispersive, acid/base surface energetics).24,38 Hence, establishing direct correlations between such complex interplay of forces and the dispersibility/aerosolisation characteristics is difficult to achieve experimentally, although it seems convincible to achieve this on theoretical principles. Interestingly, a positive correlation (R2 = 0.825) was observed between the FPF and the surface free energy of VLPP (Fig. 8). With the increase in surface energy of VLPP, aerosolisation performances were found to improve as indicated by increase in FPF. This is counterintuitive to the generally held notion that increase in surface energy promotes particle adhesion and hence hinders efficient particle dispersion. A possible explanation for such paradoxical correlation could be explained by considering the effect of aerodynamic drag exerted on the agglomerates as elucidated by following two eqn (7) and (8).
 | (7) |
 | (8) |
These equations clearly express that aerodynamic drag acting on the particles and the kinetic energy experienced by the agglomerate is directly proportional to the square and cubic of its diameter respectively. Hence, large loose aggregates formed as a result of increased cohesive forces due to higher surface energy experienced greater shear resulting in efficient de-agglomeration and hence increase in FPF. Similar results have also been obtained in some previous studies.38,39
Porosity of the VLPP governed the amount of VRZ released at the initial stage and the cumulative amount of drug released within 7 days (Fig. 9). Maximum release of VRZ was obtained in case of porous particles formulated with 5% porogen concentration as compared to VLPP formulated with 3% porogen concentration and non-porous particles. In comparison to sustained release of drugs for weeks from PLGA microspheres/nanospheres, a faster release of VRZ was observed in all the VLPP formulations. This might be due to solubility of VRZ (0.7 mg mL−1) in water and porous network within the VLPP which resulted in faster release of VRZ from VLPP.
The A549 cell viability and determination of inflammatory marker (IL-8) studies established initial safety of VRZ and VLPP (Fig. 10). These results also support the findings of the toxicity studies of inhaled VRZ solution in rats carried by Tolman et al.40 However, extensive in vivo toxicity studies are needed to be carried out to establish the long term safety of VLPP.
Uptake studies of VLPP in murine macrophages clearly demonstrated that VLPP was large enough to evade macrophage uptake and thus, ensuring prolonged residence at the site of infection (Fig. 11A). In fact, we and others have reported earlier that particles of ∼8–12 μm show very little or no uptake by macrophages, while significantly greater uptake is observed with 1–5 μm particles.20,22,41,42 The slight red florescence observed after washing of macrophages with HBSS (5×) (Fig. 11B) were most likely to be due to fragments of VLPP. However, the fraction of VLPP engulfed by macrophages was relatively small as indicated by little red florescence and its contribution to the total drug loss is expected to be insignificant. Non-porous particles, on the other hand, in the size range of 1–5 μm particles were significantly engulfed by macrophages which may constitute major portion of total drug loss (Fig. 11C and D).
Although the results demonstrate suitability of VLPP for the pulmonary delivery of VRZ, the major limitation of using large low density particles may be the increased bulk of powder to achieve therapeutically relevant dose. However, it is worth to mention that drug powder inhalers are a combination product requiring a drug formulation and device. Recently, a new inhaler device Orbital® has been designed to deliver high doses (hundreds of milligrams) of drug to the respiratory tract via multiple inhalation manoeuvres.43 Development of such devices and combining them with efficient formulations can deliver high doses of drugs such as antibiotics and antifungals to treat the infections of the lungs.
5. Conclusion
Inhaled monotherapy of VRZ as polymeric dry powder represents an attractive option for the targeted treatment of IPA. In order of achieve sustained delivery of VRZ in the lungs, VLPP were prepared and optimised using QbD principles. VLPP prepared from PLA were able to meet desirable CQAs for lung delivery in terms of physiochemical characteristics as well safety profile and macrophage uptake. Animal studies are required to further evaluate the sustained delivery of VRZ and safety following pulmonary administration of VLPP.Acknowledgements
Authors are thankful to Director, NIPER for providing necessary infrastructure facilities and for financial support.References
- T. F. Patterson, Lancet, 2005, 366, 1013–1025 CrossRef
.
- P. Venkatesan, J. R. Perfect and S. A. Myers, Dermatol. Ther., 2005, 18, 44–57 CrossRef PubMed
.
- T. J. Walsh, E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard and T. F. Patterson, Clin. Infect. Dis., 2008, 46, 327–360 CrossRef CAS PubMed
.
- D. Sheppard and L. M. Grist, Future Microbiol., 2010, 5, 1001–1004 CrossRef PubMed
.
- P. Chandrasekar, Eur. J. Haematol., 2010, 84, 281–290 CrossRef PubMed
.
- M. Karthaus and D. Buchheidt, Curr. Pharm. Des., 2013, 19, 3569–3594 CrossRef CAS
.
- O. Hilberg, C. U. Andersen, O. Henning, T. Lundby, J. Mortensen and E. Bendstrup, Eur. Respir. J., 2012, 40, 271–273 CrossRef PubMed
.
- G. F. Behre, S. Schwartz, K. Lenz, W. D. Ludwig, H. Wandt, E. Schilling, V. Heinemann, H. Link, A. Trittin and O. Boenisch, et. al., Ann. Hematol., 1995, 71, 287–291 CrossRef CAS
.
- C. A. Alvarez, N. P. Wiederhold, J. T. McConville, J. I. Peters, L. K. Najvar, J. R. Graybill, J. J. Coalson, R. L. Talbert, D. S. Burgess, R. Bocanegra, K. P. Johnston and R. O. Williams 3rd, J. Infect., 2007, 55, 68–74 CrossRef PubMed
.
- I. Bekersky, G. W. Boswell, R. Hiles, R. M. Fielding, D. Buell and T. J. Walsh, Pharmaceut. Res., 2000, 17, 1494–1502 CrossRef CAS
.
- Z. Erjavec, G. M. Woolthuis, H. G. de Vries-Hospers, W. J. Sluiter, S. M. Daenen, B. de Pauw and M. R. Halie, Eur. J. Clin. Microbiol. Infect. Dis., 1997, 16, 364–368 CrossRef CAS
.
- J. Gavalda, M. T. Martin, P. Lopez, X. Gomis, J. L. Ramirez, D. Rodriguez, O. Len, Y. Puigfel, I. Ruiz and A. Pahissa, Antimicrob. Agents Chemother., 2005, 49, 3028–3030 CrossRef CAS PubMed
.
- E. J. Ruijgrok, A. G. Vulto and E. W. Van Etten, J. Pharm. Pharmacol., 2000, 52, 619–627 CrossRef CAS
.
- J. M. Vaughn, N. P. Wiederhold, J. T. McConville, J. J. Coalson, R. L. Talbert, D. S. Burgess, K. P. Johnston, R. O. Williams 3rd and J. I. Peters, Int. J. Pharm., 2007, 338, 219–224 CrossRef CAS PubMed
.
- J. A. Tolman, N. A. Nelson, Y. J. Son, S. Bosselmann, N. P. Wiederhold, J. I. Peters, J. T. McConville and R. O. Williams 3rd, Eur. J. Pharm. Biopharm., 2009, 72, 199–205 CrossRef CAS PubMed
.
- N. A. Beinborn, H. L. Lirola and R. O. Williams 3rd, Int. J. Pharm., 2012, 429, 46–57 CrossRef CAS PubMed
.
- N. A. Beinborn, J. Du, N. P. Wiederhold, H. D. Smyth and R. O. Williams 3rd, Eur. J. Pharm. Biopharm., 2012, 81, 600–608 CrossRef CAS PubMed
.
- B. Sinha, B. Mukherjee and G. Pattnaik, Nanomed. Nanotech. Biol. Med., 2013, 9, 94–104 CrossRef CAS PubMed
.
- B. Patel, N. Gupta and F. Ahsan, J. Aerosol Med. Pulm. Drug Delivery, 2014, 27, 12–20 CrossRef CAS PubMed
.
- B. Patel, V. Gupta and F. Ahsan, J. Controlled Release, 2012, 162, 310–320 CrossRef CAS PubMed
.
- F. Ungaro, C. Giovino, C. Coletta, R. Sorrentino, A. Miro and F. Quaglia, Eur. J. Pharm. Sci., 2010, 41, 60–70 CrossRef CAS PubMed
.
- Y. Yang, N. Bajaj, P. Xu, K. Ohn, M. D. Tsifansky and Y. Yeo, Biomaterials, 2009, 30, 1947–1953 CrossRef CAS PubMed
.
- D. A. Edwards, J. Hanes, G. Caponetti, J. Hrkach, A. Ben-Jebria, M. L. Eskew, J. Mintzes, D. Deaver, N. Lotan and R. Langer, Science, 1997, 276, 1868–1871 CrossRef CAS
.
- E. Oh and P. E. Luner, Int. J. Pharm., 1999, 188, 203–219 CrossRef CAS
.
- S. Jain, D. Kumar, N. K. Swarnakar and K. Thanki, Biomaterials, 2012, 33, 6758–6768 CrossRef CAS PubMed
.
- H. Lin, H. Li, H. J. Cho, S. Bian, H. J. Roh, M. K. Lee, J. S. Kim, S. J. Chung, C. K. Shim and D. D. Kim, Journal of pharmaceutical science, 2007, 96, 341–350 CrossRef CAS PubMed
.
- S. M. Hwang, D. D. Kim, S. J. Chung and C. K. Shim, J. Controlled Release, 2008, 129, 100–106 CrossRef CAS PubMed
.
- Z. Zsengeller, K. Otake, S. A. Hossain, P. Y. Berclaz and B. C. Trapnell, J. Virol., 2000, 74, 9655–9667 CrossRef CAS
.
- S. Wold, P. Geladi, K. Esbensen and J. Öhman, J. Chemom., 1987, 1, 41–56 CrossRef CAS PubMed
.
- W. Dunn Iii, D. Scott and W. Glen, Tetrahedron Comput. Methodol., 1989, 2, 349–376 CrossRef
.
- X. Teng, J. Ren and S. Gu, J. Biomed. Mater. Res., Part B, 2007, 81, 185–193 CrossRef PubMed
.
- H. K. Kim, H. J. Chung and T. G. Park, J. Controlled Release, 2006, 112, 167–174 CrossRef CAS PubMed
.
- M. J. Kwon, J. H. Bae, J. J. Kim, K. Na and E. S. Lee, Int. J. Pharm., 2007, 333, 5–9 CrossRef CAS PubMed
.
- B. Jones and P. Goos, I-optimal versus D-optimal split-plot response surface designs, 2012 Search PubMed
.
- L. X. Yu, Pharmaceut. Res., 2008, 25, 781–791 CrossRef CAS PubMed
.
- B. Forbes, B. Asgharian, L. A. Dailey, D. Ferguson, P. Gerde, M. Gumbleton, L. Gustavsson, C. Hardy, D. Hassall, R. Jones, R. Lock, J. Maas, T. McGovern, G. R. Pitcairn, G. Somers and R. K. Wolff, Adv. Drug Delivery Rev., 2011, 63, 69–87 CrossRef CAS PubMed
.
- F. Fichtner, D. Mahlin, K. Welch, S. Gaisford and G. Alderborn, Pharmaceut. Res., 2008, 25, 2750–2759 CrossRef CAS PubMed
.
- P. Begat, D. A. Morton, J. N. Staniforth and R. Price, Pharmaceut. Res., 2004, 21, 1826–1833 CrossRef CAS
.
- R. Salama, S. Hoe, H. K. Chan, D. Traini and P. M. Young, Eur. J. Pharm. Biopharm., 2008, 69, 486–495 CrossRef CAS PubMed
.
- J. A. Tolman, N. A. Nelson, S. Bosselmann, J. I. Peters, J. J. Coalson, N. P. Wiederhold and R. O. Williams 3rd, Int. J. Pharm., 2009, 379, 25–31 CrossRef CAS PubMed
.
- C. Thomas, V. Gupta and F. Ahsan, Pharmaceut. Res., 2010, 27, 905–919 CrossRef CAS PubMed
.
- D. A. Edwards, A. Ben-Jebria and R. Langer, J. Appl. Physiol., 1998, 85, 379–385 CAS
.
- P. M. Young, J. Crapper, G. Philips, K. Sharma, H. K. Chan and D. Traini, J. Aerosol Med. Pulm. Drug Delivery, 2014, 27, 138–147 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00248f |
| This journal is © The Royal Society of Chemistry 2015 |