Effect of acidic returned fluid on the electric demulsification of crude oil emulsions
Jun Taoa,
Peng Shiab,
Shenwen Fangab,
Keyi Lia,
Ming Duan*ab and
Pingli Liuc
aSchool of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu, Sichuan 610500, People’s Republic of China. E-mail: swpua124@126.com; Fax: +8602883037346; Tel: +8602883037346
bOil & Gas Field Applied Chemistry Key Laboratory of Sichuan Province, Southwest Petroleum University, Chengdu, Sichuan 610500, People's Republic of China
cState Key Laboratory of Oil/Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu, Sichuan 610500, People’s Republic of China
First published on 23rd February 2015
Abstract
The main objective of this work is to investigate the effect of acidic returned fluid (ARF) on the electric demulsification of crude oil emulsion produced by acid fracturing technology. It was found that the pH and the iron ions of the ARF had a huge influence on the electric demulsification. The pH value could affect the strength of the interfacial film to result in the short circuit of the electric dehydrator. The short circuit only happened in basic conditions when water content (WC) was low, but when WC reached 15%, the short circuit happened both in the acidic and basic conditions. In addition, it needed less time to happen in acidic conditions due to the strength difference of the interfacial film in the acidic and basic conditions. Because of the polar interactions between asphaltenes, resins and iron ions, the iron ions had a great tendency to interact with them, thereby easily changing the adsorption structures of asphaltenes and resins on the oil–water interface to enhance the strength and thickness of the interface film, which resulted in the short circuit of the electric dehydrator.
1. Introduction
The emulsified water is generally present in crude oil as a result of the mixing occurring during production operations. These water/oil (W/O) emulsions are often stabilized by the interfacial active materials present in the crude oil, for example, asphaltenes and resins, which adsorb onto the interface and tend to inhibit or delay the inter-drop film drainage, hence preventing drop–drop coalescence and water separation. Because the crude oil emulsions increase the cost of petroleum transportation and refining, the demulsification of emulsions is essential.1–3 Nowadays, chemical demulsification is the main method for the separation of water-in-oil emulsions. The chemical agents (demulsifiers) are added to the emulsions and the natural surfactants at the interface are displaced by them, then the viscosity and elasticity of the interface film decrease, which contributes to the dehydration.4 Although most of the water in the emulsions can be removed during the chemical demulsification, the water content (WC) is too high to meet the requirements of refining. Therefore, electric demulsification is essential to decrease WC.Pioneering work on electrically enhanced demulsification was carried out by Cottrell in 1911,5,6 they just applied a high electric field onto the flowing emulsion to cause flocculation and coalescence of dispersed water droplets in the petroleum industry for separating crude oil emulsions. This technique was also applied to promote phase separation of an organic phase and aqueous dispersion, and an effective separator for solvents was developed.7,8 Allan and Mason9 studied the effect of electric field on the coalescence of water drops in a water–heptane system containing a surfactant. They found that an electric force greatly enhanced the rate of film drainage, reducing the drop lifetime. Brown and Hanson10 have also drawn similar conclusions in their study of the effect of electric field on the stability of water/kerosene emulsions. In fact, the rate of film drainage has a good correlation with the interactions between droplets, the rate of film drainage increases with the increase of the interactions. However, the interactions between droplets are rather complicated and include several forces acting on the water droplets:1,11–14 (1) dipole–dipole interactions between polarized droplets, (2) droplets in the emulsions come into contact with the electrode and acquire charge, then are repulsed by from the electrode, (3) there exist forces of gradient of non uniform electric fields, which compel droplets to move to the point of the maximum electric field intensity.
In general, an irreversible rupturing of the emulsions can occur in a high electric field due to the coalescence of droplets through these forces acting on them. In a low electric field, however, the polarized water droplets align into chains, because the electric field is not high enough to induce coalescence. When the field is switched off, the droplets return to a random distribution,15,16 the reason for this phenomenon can be explained by the classical equation
| F = 24πε0ε1r6E2/(d + 2r)4 | (1) |
Now, many oilfields of low permeability have adopted the acid fracturing method, the main function of it is to remove formation damage to enhance the productivity of the oil producing zones. Although the yield of crude oil has been greatly improved, the acidic returned fluid (ARF) produced by the technology brought about a difficult problem for the treatment of crude oil emulsions. During the chemical demulsification process, the emulsions produced with ARF usually resulted in difficult demulsification, so it may also cause some problems for electric demulsification.
For these reasons, the purpose of this paper is to investigate the effect of ARF on the electric demulsification of crude oil emulsions.
2. Experimental
2.1. Emulsion preparation
ARFs were obtained from an oilfield in China. Thirteen representational samples were collected from the same oil well at different times. The properties of the water in these samples were analyzed by us and are summarized in Table 1. Compared to the conventional produced liquid (CPL), the concentration of iron ions in the ARF emulsion is higher, and the pH value of the emulsion in the ARF is also different from the CPL.| Sample number | Iron ion (mg L−1) | pH |
|---|---|---|
| 1 | 1019.1 | 7.2 |
| 2 | 516.7 | 6.6 |
| 3 | 522.2 | 2.4 |
| 4 | 137.2 | 4.8 |
| 5 | 28.4 | 2.2 |
| 6 | 17.5 | 8.4 |
| 7 | 214.4 | 6.5 |
| 8 | 505 | 5.3 |
| 9 | 43.6 | 4.4 |
| 10 | 340.4 | 4.7 |
| 11 | 419.8 | 6.8 |
| 12 | 285.7 | 6.6 |
| 13 | 8.5 | 7.0 |
When we got the samples, the oil and water in each sample had been separated for a long time. Therefore, before the electric demulsification, the crude oil emulsions were prepared using a SG500 emulsifying machine (Shanghai ShangGui Corp.) at a rotating speed of 7000 rpm for 15 min by homogenizing known amounts of crude oil (the dielectric conductivity was about 1–2 μs cm−1) and waste water (the dielectric conductivity was about 500–1000 μs cm−1).
2.2. Bottle tests
It is well known that the main strategy of demulsification used nowadays is chemical demulsification. Therefore, before electric demulsification, chemical demulsification has been performed on the emulsions produced by the acid fracturing technology. The chemical demulsification of emulsions was conducted by bottle tests. The demulsifier was dissolved in xylene (AR) and then added to 60 mL of the previously prepared emulsions (50% (v/v) of WC) at a specific concentration (200 mg L−1). The mixture was added into 100 mL graduated bottles and then shaken vigorously for 90 s. The bottles were placed in thermostated water at 85 °C. Water separation was observed at different times.The typical ethylene oxide/propylene oxide (EO/PO) copolymer demulsifier used in the experiments was made in our laboratory. It was synthesized by propylene oxide and ethylene oxide under the conditions of high temperature and pressure. Firstly, stearyl alcohol was added into the reaction kettle to act as an initiator. Then, KOH was added into the reaction kettle to act as a catalyst. Finally, propylene oxide and ethylene oxide were added in order.
2.3. The relationship between pH, iron ions and electric demulsification
The emulsions, which contained different concentrations of iron ions (different dosages of FeCl3 were added) and different pH (HCl and NaOH were used to adjust), were prepared and used to carry out electric demulsification experiments (oil and wastewater both are from the 13# sample). In these experiments, in order to avoid the interference of WC on the electric dehydration, the WC of the emulsions is not high enough to result in the short circuit of the electric dehydrator.The electric dehydrator used in the experiment was made in our lab and the schematic diagram is shown in Fig. 1. The electric dehydrator is composed of four parts. A – Electric dehydration bottle; B – electrode; C – water bath; D – control panel. The electrodes are composed of two plates with a needle, the distance between the plates is about 15 cm and the voltage used in the experiment was maintained at 5000 V that made a strong uniform electric field form between the electrodes, which effectively avoided the forces of gradient present in non-uniform electric fields. In addition, the repulsive forces between the water droplets and the electrodes could be neglected due to the high electric field intensity. The plate is circular and the diameter is about 2.4 cm. During the experiments, the emulsions were poured into the electric demulsification bottle, and the electric demulsification temperature was 85 °C. The digital signals converted by the voltage signals will be displayed on the computer and collected by an acquisition program. Hence we can monitor the change in the current to acquire the conditions of electric demulsification, which allows us to recognize the effect of pH and iron ions on the electric demulsification.
 | ||
| Fig. 1 The schematic diagram of the electric dehydrator. A – Electric dehydration bottle; B – electrode; C – water bath; D – control panel. | ||
2.4. Oil–water interfacial tension (IFT) measurement
IFT measurements were conducted using the TX-500C ultralow interfacial tension meter (Beijing HARKE Experimental Instrument Factory). Measurements were carried out at 55 ± 1 °C using brine water (made in the lab) with different pH values (HCl and NaOH were used to adjust) as the aqueous phase and kerosene-diluted crude oil (kerosene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) crude oil = 1
crude oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)) as the oil phase. At the beginning of each measurement, a fresh oil droplet was formed at the tip of an inverted needle submerged in the aqueous phase, and monitoring of IFT was immediately initiated. The IFT was determined by using a method of successive approximation to fit a form of the Young–Laplace equation to the drop shape.
1 (v/v)) as the oil phase. At the beginning of each measurement, a fresh oil droplet was formed at the tip of an inverted needle submerged in the aqueous phase, and monitoring of IFT was immediately initiated. The IFT was determined by using a method of successive approximation to fit a form of the Young–Laplace equation to the drop shape.
2.5. The effect of pH on the stability of asphaltene-stabilized and resin-stabilized emulsions
The asphaltenes and resins, which were extracted from the crude oil, were distilled with kerosene as model oil, the concentration of asphaltene and resin were 1 wt% and 3 wt%, respectively. Emulsions were prepared by using the brine water with different pH values and model oil (model oil and wastewater in the ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)). For 25 min, the stabilities of the emulsions were investigated according to the change in the conductivity of the emulsions by the digital conductivity detector (Shanghai Jin instrument Co. Ltd). The experiments were conducted at 25 °C ± 0.1 °C.
3 (v/v)). For 25 min, the stabilities of the emulsions were investigated according to the change in the conductivity of the emulsions by the digital conductivity detector (Shanghai Jin instrument Co. Ltd). The experiments were conducted at 25 °C ± 0.1 °C.
2.6. Determination of the partition coefficient of the iron ions at the oil phase and water phase, and the existing state of iron ions in crude oil
The emulsions with known concentrations of iron ions were (wastewater and oil are from the 13# sample) prepared for chemical demulsification. The concentration of iron ions in the free water was detected by the atomic absorption spectrophotometer (AAS, Beijing East & West instrument analysis Co.), which was used together with the initial concentration of iron ions at the water to confirm the partition coefficient of the iron ions.The existing state of a metal in crude oil is basically divided into three categories: inorganic salt, organic salt and complexes with large molecular weights. The inorganic salt and organic salt can be removed by distilled water and acidic water respectively. The complexes need to be separated from crude oil and purified by solvent extraction. The operations were as follows:
The process of acidic water extraction was similar to the distilled water extraction, just replacing the distilled water and acetone with a 1% sulfuric acid solution.
2.7. Determination of the interactions between iron ions and the saturates, aromatics, resin and asphaltene
For a better understanding of the interactions between iron ions and crude oil, the four components of crude oil were separated and distilled with kerosene as the oil phase, and the wastewater of the 13# sample containing different content of iron ions as the water phase. They were employed to prepare emulsions (model oil and wastewater in the ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)). Their stabilities were investigated according to the change in the conductivities of the emulsions measured by the digital conductivity detector. The experiments were conducted at 25 °C ± 0.1 °C. The conductivities of the oil and water phases were measured.
3 (v/v)). Their stabilities were investigated according to the change in the conductivities of the emulsions measured by the digital conductivity detector. The experiments were conducted at 25 °C ± 0.1 °C. The conductivities of the oil and water phases were measured.
3. Results and discussion
3.1. The effect of the demulsifiers on the demulsification of emulsions
The effect of the demulsifiers on the demulsification is shown in Table 2. The WC range of the emulsions after demulsification was from 6.2% to 21.0%. In general, the water level above 0.5% constitutes a quality problem and a concomitant price reduction,21 so it is essential to conduct electric demulsification of the emulsions. However, as we mentioned above, the emulsions produced by acid fracturing technology may bring some problems for electric demulsification, so the effect of AFR on the electric demulsification should be investigated.| Number | Concentration of demulsifier (mg L−1) | Dehydration time (min) and dehydration ratio (%) | WC% | ||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 60 | |||
| 1 | 200 | 50.0 | 56.7 | 56.7 | 67.0 | 73.3 | 21.0 |
| 2 | 200 | 32.0 | 43.0 | 73.3 | 73.3 | 80.0 | 16.7 |
| 3 | 200 | 32.0 | 43.0 | 56.7 | 73.3 | 76.7 | 18.9 |
| 4 | 200 | 23.3 | 33.3 | 50.0 | 66.7 | 83.3 | 14.3 |
| 5 | 200 | 10.0 | 23.3 | 63.3 | 76.7 | 80.0 | 16.7 |
| 6 | 200 | 80.0 | 90.0 | 90.0 | 92.0 | 93.3 | 6.2 |
| 7 | 200 | 80.0 | 83.3 | 83.3 | 86.7 | 90.0 | 9.1 |
| 8 | 200 | 23.3 | 56.7 | 67.0 | 76.7 | 80.0 | 16.7 |
| 9 | 200 | 56.7 | 56.7 | 76.7 | 82.0 | 86.7 | 11.8 |
| 10 | 200 | 56.7 | 70.0 | 73.3 | 76.7 | 80.0 | 16.7 |
| 11 | 200 | 40.0 | 60.0 | 73.3 | 80.0 | 83.3 | 14.3 |
| 12 | 200 | 76.7 | 76.7 | 86.7 | 86.7 | 93.3 | 6.2 |
| 13 | 200 | 83.3 | 86.7 | 90.0 | 93.3 | 93.3 | 6.2 |
3.2. The effect of pH on the electric dehydration
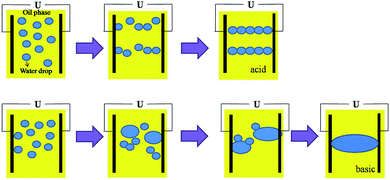
The WC of the emulsions used in the electric demulsification process was kept at 10%. Fig. 2 shows the effect of pH on electric dehydration. When the pH was below 7, the current change went from violent to gentle with the increase of pH, while the current became violent again when the pH exceeded 7. The short circuit did not occur when the pH was acidic, however it happened when the pH was greater than 7. The result indicated that the pH value had an evident influence on the conductivity during the electric dehydration process.In general, the interfacial tension (IFT) can reflect the adsorption of natural emulsifiers at the oil–water interface. The lower the IFT is, the more the emulsifier adsorbs onto the oil–water interface until saturation is achieved. Fig. 3 shows the effect of pH on the IFT. When the pH was set at 7, the IFT reached a maximum, and decreased when the pH increased or decreased, indicating that a rigid film can be formed in the acidic or alkaline conditions. It has been reported that when two drops were surrounded by a rigid film, stable chains would be created that lead to bridging between the electrodes, eventually resulting in short circuit,11,22,23 therefore, the short circuit should also happen in the acidic conditions, but it was not observed in the experiments.
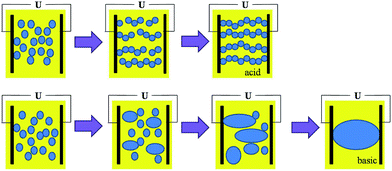
Fig. 4a–c shows the effect of pH on the stabilities of asphaltene-stabilized and resin-stabilized emulsions, there was a correlation between the stabilities of the emulsions and the pH range. Fig. 4a and b indicates that the stabilities of the asphaltene-stabilized emulsions decreased as the pH increased, when the pH varied from 2 to 10, the conductivity of the emulsions varied from 0.21–1.8 μs cm−1. Fig. 4c demonstrates that the stabilities of the resin-stabilized emulsions increased with the increase of the pH value. At pH 2, the oil and water completely separated after 1 min, at pH 10, the emulsions were still relatively stable after 25 min. Therefore, it is known that the pH has a huge influence on the strength of the interface film, the rigid interfacial film formed by asphaltenes are strongest in acidic pH, intermediate in strength at neutral pH and become weak in basic pH; the interfacial film produced by the resins are strongest in base and weakest in acid, which is consistent with the results of Strassner.24 So when the pH decreases or increases, though the IFT decreases in both cases, the strength of the film is different. The film is not easy to rupture in the acidic range, and easy to rupture in the basic range under high voltage electric demulsification because the strength of the film formed by the asphaltene is stronger than the film formed by resin due to its self-aggregation to form clusters on the interface.25–27 Fig. 4a and c also confirmed this point, the asphaltene-stabilized emulsions were more stable than the resin-stabilized emulsions. Hence it is easy to form water chains in acidic conditions and cause the coalescence of drops in basic conditions which results in the differences between electric demulsifications under different conditions. However, when the WC is low, the number of water chains in the acidic pH is not enough to make the conductivity exceed the capacitance. In contrast, a lot of water causes coalescence to form a “waterway” in the basic conditions and makes the conductivity exceed the limit, hence the short circuit happened, a schematic diagram of this is presented in Fig. 5. Meanwhile, it is easy to conclude that when the WC is higher, although the short circuit happened in both environments due to the high conductivity, less time was needed for the short circuit to occur in acidic pH because the formation of water chains was prior to the coalescence. The schematic diagram of this is shown in Fig. 6.
 | ||
| Fig. 4 Effect of pH on the stability of model emulsions (a, b – asphaltenes-stabilized emulsions; c – resins-stabilized emulsions). | ||
Fig. 7 shows the synergistic effects of WC and pH. When the WC exceeded 15%, the short circuit happened in both conditions. It was obvious that the results were consistent with the conclusion, the short circuit in the acidic range needed less time than in the basic range, which confirmed that the water chain formed in the acidic pH, and the coalescence of water droplets occurred in the basic pH.
3.3. The effect of iron ions on electric dehydration
The effect of different concentrations of iron ions on the electric dehydration of emulsions (WC is 10%) is shown in Fig. 8a and b. It is observed that the current of the whole electric dehydration process changed more and more intensely with the increase in the concentration of iron ions. When the iron ions increased to 1000 mg L−1, the current reached a maximum and eventually the short circuit happened. The effect of iron ions on the conductivity of the emulsions was investigated and the results are shown in Fig. 9. It was found that the conductivity of the emulsion was still low compared to the formation of water (500–1000 μs cm−1) when the concentration of iron ions was in the range 200 mg L−1 to 1200 mg L−1. Hence the conductivity change from the increase of iron ions didn’t influence the electric dehydration. | ||
| Fig. 8 The effect of the concentration of iron ions in the ranges (a) 200 mg L−1 to 500 mg L−1 and (b) 800 mg L−1 to 1200 mg L−1 on the electric dehydration. | ||
Therefore we suggested it might be the interactions between iron ions and crude oil which result in the short circuit. For this reason, the concentration of iron ions in the free water after demulsification was also researched. As shown in Table 3, the concentration of iron ions in the free water decreased a lot, which indicated a portion of the iron ions transferred from the water phase to the oil phase and interacted with the oil. The iron ions in the crude oil were extracted and the results are shown in Table 4. Most of the iron ions were removed from the crude oil after extraction by acidic water, just a small proportion were removed by the distilled water and acetonitrile, it was demonstrated that the existing state of iron ions in crude oil was mainly polymer complexes and the short circuit may have resulted from these.
| Initial concentration of iron ions in water (mg L−1) | 200 | 300 | 500 | 800 | 1000 | 1200 |
| Final concentration of iron ions in water (mg L−1) | 33.27 | 60.39 | 119 | 159.7 | 186 | 208 |
| Residual rate of iron ions in water (%) | 16.64 | 20.13 | 23.8 | 19.96 | 18.6 | 17.33 |
| Number | Content of iron ions (mg L−1) | ||||||
|---|---|---|---|---|---|---|---|
| Initial concentration of iron ions in water | 0 | 200 | 300 | 500 | 800 | 1000 | 1200 |
| Iron ions in the oil after extraction by distilled water | 5.8 | 173.5 | 274.1 | 481.6 | 773 | 983.4 | 1137.2 |
| Iron ions in the oil after extraction by acidic water | 1.5 | 5.7 | 8.4 | 34.2 | 69.4 | 134.1 | 184.0 |
| Iron ions in the oil after extraction by acetonitrile | 2.5 | 182.4 | 269.0 | 484.4 | 768.3 | 978.9 | 1164.3 |
3.4. Interactions between the iron ions and the saturates, aromatics, asphaltenes and resins
There were polar interactions between the asphaltene, the resin and the metal ions,28–31 and the interactions are presented as follows:31| ΔGTot132 = ΔGLW132 + ΔGAB132 + ΔGEL132 + … | (2) |
The ΔGLW132 and ΔGAB132 can be calculated from the following equations, respectively.
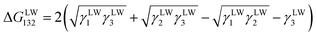 | (3) |
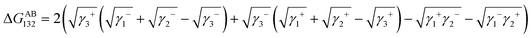 | (4) |
In order to understand the polar interactions, the interactions between iron ions and the four components of crude oil were investigated by measuring the change in conductivity, and the results are shown in Fig. 10a–d. Fig. 10a and b show that the conductivities of the saturate and the aromatic components with different concentrations of iron ions didn’t exhibit any differences compared to the blank sample, which illustrated that the iron ions don’t have any influence on them. However, Fig. 10c and d show that the conductivities of the asphaltene and the resin changed slowly. Especially for the asphaltene, compared to the blank, the change in the conductivity of the model oil was slower.
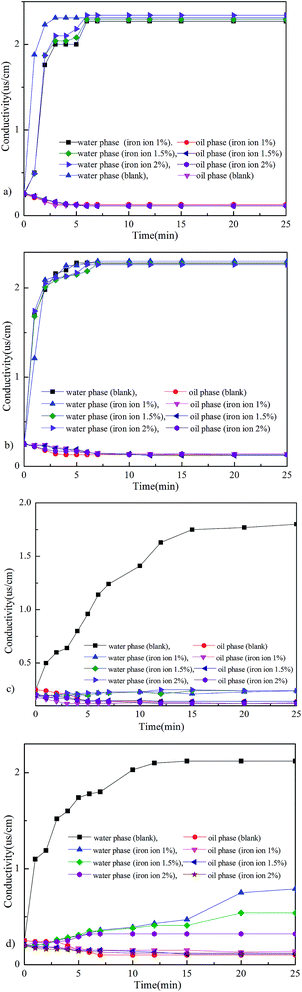 | ||
| Fig. 10 The interactions between iron ions and the four components of crude oil (a – saturate; b – aromatic; c – asphaltene; d – resin). | ||
According to the results (Fig. 10c–d), it was obvious that the interaction between asphaltenes, resins and iron ions was spontaneous and these results are similar with the study by Xie et al.30 The reason for these polar interactions is the heteroatom (such as O and N) and the aromatic rings of the asphaltenes and resins interacted with the iron ions, which resulted in ΔGTot132 < 0.
Due to the forces between the asphaltene, or the resin and the iron ions, the iron ions had a great tendency to interact with them, thereby easily changing the adsorption structure of the asphaltene and the resin on the oil–water interface to enhance the strength and thickness of interface film. For the asphaltene, the change in conductivity was more obvious than for the resin, this could be attributed to the easier formation of asphaltene aggregates at the interface,25,27 which made the asphaltene model oil more stable. So the change in the conductivity of the asphaltene model oil was slower than the resin’s.
The overall results showed that the interface film became stronger with the increase of iron ions to hinder the drop–drop coalescence which was the main reason for the occurrence of a short circuit. This made the number of water chains increase, hence the conductivities and currents of the emulsions increased until the short circuit of the electric dehydrator.
4. Conclusions
The pH and concentration of iron ions of ARF both had an influence on the electric demulsification of crude oil emulsions:(1) The chemical demulsification of emulsions produced by acid fracturing technology was difficult. The WC range of the emulsions after demulsification were in the range 6% to 21%, which were still relatively high compared to the requirements of the oil refinery.
(2) The strength of the interfacial film was closely connected with the pH scale, it was strong in the acidic pH and weak in the basic pH, which made the formation of water chains easy in the acidic pH and the coalescence of drops easy in the basic pH. When the WC of emulsions was low, the conductivity caused by the water chains was not enough to result in a short circuit in the acidic conditions. However, a lot of water coalesced to form a “waterway” in the initial stage of electric dehydration, which made the conductivity exceed the capacitance between the electrodes, hence resulting in a short circuit. The short circuit also happened when the WC was above 15%, it just needed less time to happen in the acidic conditions, because the formation of water chains was prior to the coalescence of drops.
(3) With the increase of iron ions, the current also increased. The main factor for a short circuit was the interactions between the asphaltene, or the resin and the iron ions. Because of the heteroatom (such as O and N) and aromatic ring of the asphaltene and the resin, polar interactions (such as Lifshitz–van der Waal (LW) interactions, acid–base (AB) interactions and electrostatic interactions) were formed. This meant that the iron ions had a great tendency to interact with them to increase the strength of the interfacial film, which made the water chains form easily. For asphaltene, the change in conductivity was more obvious than for the resin, this could be attributed to the easier formation of asphaltene aggregates at the interface.
Acknowledgements
The authors gratefully thank the support provided by the Science-Technology Foundation for Young Scientist from the Science and Technology Department of Sichuan Province (2013JQ0009). The support from the Applied Basic Research Programs of the Science and Technology Department of Sichuan Province (2014JY0120) is also thanked.References
- R. A. Mohammed, A. I. Bailey, P. F. Luckham and S. E. Taylor, Colloids Surf., A, 1994, 83, 261 CrossRef CAS.
- R. C. B. Lemos, E. B. da Silva, A. d. Santos, R. C. L. Guimarães, B. M. S. Ferreira, R. A. Guarnieri, C. Dariva, E. Franceschi, A. F. Santos and M. Fortuny, Energy Fuels, 2010, 24, 4439 CrossRef CAS.
- M. A. Krawczyk, D. T. Was and C. Shetty, Ind. Eng. Chem. Res., 1991, 30, 367 CrossRef CAS.
- W. L. Kang, G. L. Jing, H. Y. Zhang, M. Y. Li and Z. L. Wu, Colloids Surf., A, 2006, 272, 27 CrossRef CAS PubMed.
- F. G. Cottrell, US Pat. 994377, 1911 Search PubMed.
- F. G. Cottrell and J. B. Speed, US Pat. 987114, 1911 Search PubMed.
- P. J. Bailes and S. K. L. Larkai, Chem. Eng. Res. Des., 1981, 59, 229 CAS.
- P. J. Bailes and S. K. L. Larkai, Chem. Eng. Res. Des., 1982, 60, 115 CAS.
- R. S. Allan and S. G. Mason, Trans. Faraday Soc., 1961, 57, 2027 RSC.
- A. H. Brown and C. Hanson, Trans. Faraday Soc., 1965, 61, 1754 RSC.
- S. E. Taylor, Colloids Surf., 1988, 29, 29 CrossRef CAS.
- R. S. H. Kashaev, Advances in Energy Research, Inc. ID 12590, N-Y USA 1, vol. 16, 2013 Search PubMed.
- R. S. H. Kashaev, Author’s certificate 1333364, 1985.
- R. S. H. Kashaev and N. R. Faskhiev, Appl. Magn. Reson., 2011, 41, 31 CrossRef.
- D. Sun, X. Duan, W. Li and D. Zhou, J. Membr. Sci., 1998, 146, 65 CrossRef CAS.
- H. Brandenberger, D. Nussli, V. Piech and F. Widmer, J. Electrost., 1999, 45, 227 CrossRef CAS.
- T. J. Williams and A. G. Bailey, IEEE Trans. Ind. Appl., 1986, 1A-22, 536 CrossRef.
- P. J. Bailes and E. H. Stitt, Chem. Eng. Res. Des., 1987, 65, 514 CAS.
- L. C. Waterman, Chem. Eng. Prog., 1965, 61, 51 CAS.
- P. J. Bailes and S. K. L. Larkai, Chem. Eng. Res. Des., 1984, 62, 33 CAS.
- M. Chiesa, S. Ingebrigtsen, J. A. Melheim, P. V. Hemmingsen, E. B. Hansen and Ø. Hestad, Sep. Purif. Technol., 2006, 50, 267 CrossRef CAS PubMed.
- S. E. Taylor, Inst. Phys. Conf. Ser., 1991, 118, 185 CAS.
- T. Y. Chen, R. A. Mohammed, A. I. Bailey, P. F. Luckham and S. E. Taylor, Colloids Surf., A, 1994, 83, 273 CrossRef CAS.
- J. E. Strassner, Soc. Pet. Eng. J., 1968, 20, 303 CAS.
- L. X. Xia, S. W. Lu and G. Y. Cao, J. Colloid Interface Sci., 2004, 271, 504 CrossRef CAS PubMed.
- Y. R. Fan, S. Simon and J. Sjöblom, Colloids Surf., A, 2010, 366, 120 CrossRef CAS PubMed.
- O. León, E. Rogel, J. Espidel and G. Torres, Energy Fuels, 2006, 14, 6 CrossRef.
- D. Dudášová, S. Simon, P. V. Hemmingsen and J. Sjöblom, Colloids Surf., A, 2008, 317, 1 CrossRef PubMed.
- R. M. Balabin, R. Z. Syunyaev, T. Schmid, J. Stadler, L. R. Ekaterina and R. Zenobi, Energy Fuels, 2011, 25, 189 CrossRef CAS.
- K. Xie and K. Karan, Energy Fuels, 2005, 19, 1252 CrossRef CAS.
- C. J. van Oss, Interfacial Forces in Aqueous Media, Marcel Dekker, Inc., New York, 1st edn, 1994 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |