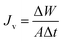DOI:
10.1039/C5RA00182J
(Paper)
RSC Adv., 2015,
5, 38011-38020
Performance evaluation of novel PVDF–Cloisite 15A hollow fiber composite membranes for treatment of effluents containing dyes and salts using membrane distillation
Received
5th January 2015
, Accepted 21st April 2015
First published on 21st April 2015
Abstract
The present study reports the performance of a novel PVDF–Cloisite 15A hollow fiber composite membrane for the treatment of effluents containing dyes and salts through a direct contact membrane distillation (DCMD) process. The performance of the membrane was evaluated by varying several important parameters during the DCMD process which included feed properties (type of dyes, dye and salt concentration) and process conditions (feed temperature and flow rate). The experimental results showed that the in-house made membrane was able to achieve stable fluxes and excellent dye rejections (>97%) when tested with feed solutions containing dyes of different classes and molecular weights (MW), except crystal violet (CV) dye. The lower rejection resulting from a CV-containing feed is likely due to its small MW coupled with its high diffusion rate in aqueous solution. With respect to feed concentration, it is found that an increase in salt concentration in the feed solution had a negligible effect on the membrane separation performance. Increasing the dye concentration in the feed however led to lower membrane water flux owing to the deposition of dye particles on the membrane surface which resulted in severe fouling. Meanwhile, increasing the feed temperature and its flow rate could improve the membrane flux without affecting the permeate quality. When tested using a dyeing solution containing 50 ppm acid red and 1.0 M NaCl, the membrane flux was reported to enhance by 200% and 25% on increasing the feed temperature from 50 to 90 °C and the flow rate from 0.010 to 0.023 m s−1, respectively.
1 Introduction
Wastewater treatment has been a challenge for the textile and dyestuff manufacturing industries due to the color exhibited by the effluents. Colored textile effluents usually represent severe environmental problems as they contain a mixture of chemicals, auxiliaries and dyestuffs of different classes and chemical constitutions with elevated organic and inorganic parameters.1–3 The colored textile effluents often come from printing and dyeing processes that use high concentrations of dyes, additives and salts to produce high quality fabrics.4 Synthetic dyes, which are the most common among all other dyes, are typically used in textile industries. They are generally derived from coal tar and petroleum based intermediates.5,6 According to Sen and Demirer,6 more than 7 × 107 tons of synthetic dyes are produced and consumed worldwide annually. Of these synthetic dyes, azo dyes are the largest and the most common group of dyes used in the textile industry.1,7 However, it must be noted that azo dyes are highly toxic, mutagenic and carcinogenic in nature.8 The presence of a small quantity (even at 1 ppm level) of azo dyes in water can be visible and results in an acute effect to the aquatic system due to their high level of toxicity.2,9
Owing to ecological factors, a new global trend on developing various sustainable technologies for removal of such colored agents from aqueous solutions is of significant environmental and technical importance. The technologies that have been employed for textile effluent treatment can be categorized based on chemical, physical and biological methods.10–12 Some of the examples of physical–chemical treatment methods are membrane filtration, coagulation/flocculation, precipitation, flotation, adsorption, ion exchange, electrolysis, advanced oxidation and chemical reduction. With respect to biological methods, bacterial/fungal biosorption and biodegradation in aerobic, anaerobic, anoxic or combined aerobic/anaerobic conditions have been generally reported.10 Although physical–chemical methods are a good option regarding high color and suspended substances removal, they are associated with some problems such as sludge generation, low chemical oxygen demand (COD) removal and high operating cost. Biological treatment process on the other hand experiences several technical challenges such as difficulty of maintaining bacterial growth, longer period of treatment cycle, etc.13
To date, the importance of membrane technologies in textile wastewater treatment is continuously growing. Membrane separation processes have become the best alternative methods that can be adopted for large-scale treatment process owing to the advantages such as environmentally friendly, high removal efficiency, modest energy requirement, etc.14 Among the membrane-based processes, membrane distillation (MD) has been seen as a potential candidate in treating textile effluents as this membrane process can be operated at very low pressure, thus minimizes fouling effect.15–23 Furthermore, MD can exploit the free energy given by hot effluent discharged by textile industry.16,23 In order to consistently maintain the effluent temperature during treatment process, low-grade waste and/or alternative energy sources such as solar and geothermal energy can be potentially integrated with MD process.24 Another promising feature of MD is its ability to reject 100% (theoretically) ions, macromolecules, colloids, cells, and other non-volatile organic compounds from the wastewater as its separation mechanism is mainly governed by vapor–liquid equilibrium (VLE).25
In our previous work,21 we have investigated the effect of Cloisite 15A clay loadings (zero–10 wt%) on the properties of polyvinylidene-fluoride (PVDF)-based hollow fiber membranes for MD application. Of the PVDF–Cloisite 15A composite membranes studied, it is found that the incorporation of 3 wt% Cloisite 15A was the ideal loading to produce best performing composite membrane by taking into consideration the membrane structural properties and separation characteristics. In this work, we will further evaluate the potential of this membrane in treating feed solutions containing dyes and salts via direct contact membrane distillation (DCMD) system. The separation performances of this membrane will be studied under different conditions by varying the properties of feed solution as well as process conditions.
2 Experimental
2.1 Materials
Five synthetic dyes supplied by Sigma-Aldrich were used as received and their classification and chemical structure are shown in Fig. 1 and Table 1, respectively. Salt, sodium chloride (NaCl, MW = 58.44 g mol−1) supplied by Merck was added to the dyeing solution to simulate industrial textile wastewater which often contains dissolved salts in the effluent.
 |
| | Fig. 1 Chemical structure of the synthetic dyes used in this work. | |
Table 1 Overview of dye classification used in this study1,26
| Class |
Principal substrates |
Method of application |
Chemical types |
Dye fixation (%) |
Dyes used in this study |
| Acid |
Nylon, wool, silk, inks, leather |
Usually from neutral to acidic dyebaths |
Azo (including premetallized), anthraquinone, triphenylmethane, azine, xanthene, nitro and nitroso |
89–95 |
Acid red 1 (AR1), congo red (CR) |
| Basic |
Polyacrylonitrile, modified nylon, polyester, inks |
Applied from acidic dyebaths |
Cyanine, hemicyanine, diazahemicyanine, diphenylmethane, triarylmethane, azo, azine, xanthene, acridine, oxazine, anthraquinone |
95–100 |
Crystal violet (CV) |
| Reactive |
Cotton, wool, silk, nylon |
Reactive site on dye reacts with functional group on fiber to bind dye covalently under influence of heat and pH (alkaline) |
Azo, anthraquinone, phthalocyanine, formazan, oxazine, basic |
50–90 |
Reactive orange 16 (RO16), reactive black 5 (RB5) |
2.2 Stokes diameter of dye particle
The diameter, dA (nm) of the dye particle was measured using Stokes–Einstein equation:27| |
 | (1) |
where k is the Boltzmann coefficient (J/K), T is temperature (K), μB is the water viscosity (Pa s) and DAB is the diffusion coefficient (m2 s−1) of dye in water. The DAB can be further defined according to the Wilke–Chang equation:| |
 | (2) |
where ϕ is the water association parameter, MB is the MW of water (g mol−1), μB is the water viscosity (Pa s) and VA is the molar volume of dye particle (m3 kg−1 mol−1). The molar volume for each dye was calculated using a group contribution method.28
2.3 Membrane fabrication
PVDF–Cloisite 15A dope solution consisted of 12 wt% PVDF and 3 wt% Cloisite 15A (i.e. clay concentration was determined based on the total weight of PVDF) was prepared by dissolving the PVDF pellets and clay powder in the NMP (80 wt%) and EG (8 wt%) mixture, while stirring at 60 °C, until the solution became homogeneous. After that, the PVDF–Cloisite 15A hollow fiber composite membrane was fabricated using dry-jet wet spinning technique in which the detailed fabrication procedure can be found in our previously published work.21 After air-drying process, the membrane was subject to several characterizations to determine its structural properties.
2.4 Direct contact membrane distillation (DCMD) experiments
A stainless steel module containing PVDF–Cloisite 15A hollow fiber composite membrane was prepared and used to determine the performances of the membranes during DCMD process. Table 2 shows the details of the membrane properties and its module. The DCMD system that was used in this work is illustrated in Fig. 2, together with SEM images of the hollow fiber composite membrane. The system was designed to have two circulating streams, i.e. hot stream also known as feed stream (circulated through the membrane shell-side) and cold stream (fed through the lumen-side of the hollow fiber membrane). Both solution temperatures were controlled using coiled heater (830, PROTECH) and chiller (F26-ED, JULABO), respectively. The change of feed concentration in the feed tank during experiment was assumed to be negligible as large feed volume (5 L) was used. In order to avoid membrane flux decline caused by dye deposition (fouling), a new membrane module was used whenever there was a change in the feed property and/or process condition.
Table 2 Membrane properties and module design
| Membrane properties |
| Average pore size (nm) |
88 |
| Porosity (%) |
83.70 ± 0.67 |
| Contact angle (°) |
97.72 ± 2.54 |
| Fiber outer dia. (μm) |
763 ± 19 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Module design |
| Module inner dia. (m) |
0.01 |
| Module length (m) |
0.22 |
| Number of fibers in module |
20 |
| Effective fiber length in module (m) |
0.19 |
| Effective membrane area in module (m2) |
0.01 |
 |
| | Fig. 2 Schematic DCMD experimental setup and the SEM micrographs of PVDF–Cloisite 15A hollow fiber composite membrane, (a) cross sectional view (magnification of 150×), (b) outer surface (magnification of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×) and (c) inner surface (magnification of 10 000×) and (c) inner surface (magnification of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). 000×). | |
Prior to the dyeing solution treatment process, the permeate flux, Jv of membrane (kg m−2 h−1) was determined using eqn (3).
| |
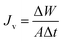 | (3) |
where Δ
W (kg) is the weight of permeate collected over a predetermined time Δ
t (h) of process and
A (m
2) is the effective membrane area. To determine the solute (dye or salt) rejection,
R (%) of the membrane,
eqn (4) was employed.
| |
 | (4) |
where
Cp and
Cf stand for permeate and feed concentration (mg L
−1), respectively. The results of flux and rejection reported in this work were the average of three measurements.
2.5 Analytical methods
2.5.1 Dye and salt concentration determination. The dye concentration in the sample solutions was detected by a UV-vis spectrophotometer (DR5000, Hach) which measured at its maximum absorbance wavelength. Meanwhile, the ionic conductivity of the sample solutions was measured using a bench conductivity meter (4520, Jenway). Both sample absorbance and conductivity were later converted into concentration using a calibration curve.
2.5.2 SEM-EDX. The dry membrane samples were immersed in liquid nitrogen and fractured, followed by sputter-coating with platinum using a sputtering device. The cross-sections of the membrane samples were examined using scanning electron microscope (SEM) (TM-3000, Hitachi). Meanwhile, energy-dispersive X-ray (EDX) spectrometer (XFlash® 430H, Bruker) was used for elemental analysis in order to identify the elements caused by foulants that deposited on membrane surface.
3 Results and discussion
3.1 Effect of dye characteristics on DCMD performance
Fig. 3 shows the effect of dye characteristics on the permeate flux and rejection of PVDF–Cloisite 15A hollow fiber composite membrane during DCMD process. The error bars indicate the standard deviations of the average measured values of both water flux and dye rejection. From the figure, it can be clearly seen that the in-house made membrane could achieve very similar permeate fluxes (around 10 kg m−2 h−1) for all dyes studied, except for CV which showed >17 kg m−2 h−1. With respect to separation characteristics, it is found that the membrane could potentially eliminate almost all types of dyes regardless of their MW and Stokes diameter. Although the membrane average pore size (dp = 88 nm) is significantly larger than those of dyes' particle size (Table 3), its excellent separation performance is not compromised. This is because MD does not work according to the principle of size exclusion and/or Donnan exclusion as in ultrafiltration and nanofiltration.29–32 It instead works as a physical barrier to hold the liquid–vapor interface at the entrance of the membrane pores. In view of this, the separation mechanism in MD is predominantly determined by the VLE principle.33
 |
| | Fig. 3 Effect of dye components on the permeate flux and dye rejection of membrane during DCMD process (conditions = hot stream: 50 ppm dyeing solution, 70 °C at flow rate of 0.023 m s−1; cold stream: distilled water, 20 °C at flow rate of 0.010 m s−1). | |
Table 3 Properties of synthetic dyes used in this work
| Dye |
aλmax (nm) |
bMW (g mol−1) |
cDAB (10−10 m2 s−1) |
ddA (nm) |
| λmax = maximum absorbance wavelength. MW = molecular weight. DAB = diffusion coefficient of dye in water. dA = stokes diameter of dye in water. |
| CV |
590 |
407.98 |
3.99 |
1.23 |
| AR1 |
506 |
509.42 |
4.25 |
1.15 |
| RO16 |
493 |
617.54 |
3.81 |
1.29 |
| CR |
498 |
696.66 |
3.24 |
1.52 |
| RB5 |
597 |
991.82 |
3.01 |
1.63 |
Unlike other dye components which displayed very similar flux and rejection results, the CV dye seemed to have different interaction with the membrane matrix, leading to relatively higher permeate flux but lower removal rate. This can be possibly due to the high “affinity” of this particular dye towards the membrane matrix. The high adsorption rate of CV towards PVDF-based membrane is believed to be the main factor changing the color of membrane after treatment process (see Fig. 4(a)). Furthermore, the high diffusivity of CV in aqueous solution could be another reason causing less promising dye removal (<98%) as evidenced from the permeate sample collected (Fig. 4(b)). The detection of nitrogen (N) and chlorine (Cl) element on the composite membrane surface as shown in Fig. 5 strongly indicates the presence of dye component on the membrane surface, owing to the possible interaction between the aromatic rings of the dye molecule and the membrane via van der Waals.22
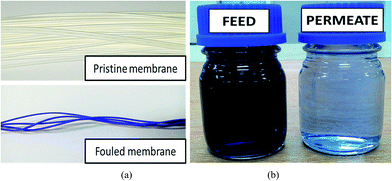 |
| | Fig. 4 Direct comparisons between (a) pristine membrane and fouled membrane and (b) 50 ppm CV dyeing solution (feed) and permeate produced by the composite membrane. | |
 |
| | Fig. 5 EDX results on the membrane surface after treating CV dyeing solution. | |
3.2 Effect of dye concentrations on DCMD performance
In general, approximately 10–20% of textile dyes are lost during dyeing process and as a consequence, the effluent discharged typically contains between 10 and 1000 ppm of dye components.34,35 To evaluate the effect of dye concentration on the DCMD performance, a series of experiments were carried out and the results are shown in Fig. 6. Results showed that both permeate flux and dye rejection tended to decrease with increasing dye concentration from 50 to 1000 ppm. The permeate flux decline at high solute concentration can be caused by the lower water vapor transport rate. This phenomenon is common in MD process as higher solute concentration could lead to lower activity coefficient of water vapor pressure.33,36
 |
| | Fig. 6 Permeate flux and dye rejection as a function of dye concentration (conditions = hot stream: 70 °C at flow rate of 0.023 m s−1; cold stream: distilled water, 20 °C at flow rate of 0.010 m s−1). | |
In addition to activity coefficient of water vapor pressure, the reduction of permeate flux and dye rejection at high concentration of dye solution is also possibly due to the attachment of dye particles on the membrane surface which leads to either partially or fully pore blockage. Additional fouling layer could be developed which further reduces the evaporation area and affects permeate quantity. As reported by Yarlagadda et al.,37 severe fouling caused by high feed concentration may damage the membrane surface and allow the passage of small quantities of non-volatile solutes through the membrane. In addition, increasing feed concentration will also increase feed viscosity and boundary layer thickness, which enhances the mass transfer resistances.33 Even though MD experienced lower permeate flux at high dye concentration, its degree of flux decline was still much lower compared to pressure-driven membrane process, e.g. nanofiltration. Comparing the results obtained from lowest and highest dye concentration, it is found that the flux of MD was only reduced by 12.4% with dye rejection maintained at >90%.
3.3 Effect of salt concentrations on DCMD performance
Cotton is the most important and widely used textile fiber in the world which consists around 88–96% of pure cellulose.38 However, natural cellulose fibers commonly carry negative charge, which create repulsion with anionic dyes. In order to promote dye–fiber fixation, high amount of NaCl is needed to suppress the fiber surface charge. This as a consequence has led the effluent to have high NaCl concentration (0.7–1.4 M).39 In this section, the effect of feed salt concentration on the performance of DCMD process was investigated and the results are shown in Fig. 7. It can be seen from the figure that the variation in NaCl concentration in the feed solution has very little impact on the performance of membrane with respect to permeate flux and separation characteristics. The findings from this work were consistent with Banat et al.15 in which they also reported that salt concentration (0.05–1.0 M) has negligible effect on the driving force for the vapor flux.
 |
| | Fig. 7 Permeate flux and solute rejection as a function of salt concentration in the dyeing solution containing 50 ppm ARI (conditions = hot stream: 70 °C at flow rate of 0.023 m s−1, cold stream: 20 °C at flow rate of 0.010 m s−1). | |
Although the salt concentration has negligible effect on membrane permeate flux, it does have huge impact on membrane scaling when it is present at high concentration. Fig. 8 shows the SEM images of the membranes after testing with two different salt concentrations (0.1 and 1.0 M). Crystallized salts were found within the structure of the fouled membrane after treating 1.0 M NaCl feed solution. This observation could be directly related to membrane scaling.40 Generally, the scaling occurs when the salt concentration in the feed solution reaches supersaturation due to high product water recovery. Concentration and temperature polarizations are the major causes for the salt solution to become supersaturated and crystallize directly on the membrane surface or crystallize in the bulk solution and deposit on the membrane surface. The presence of salt on the composite membrane was further analysed based on EDX results shown in Fig. 9. As can be seen, Na and Cl elements corresponded to salt were detected, in addition to the elements (F, C, O, Si and Al) belonging to the PVDF–Cloisite 15A membrane. Furthermore, the amounts of Na and Cl detected are consistent with the SEM images shown. Since the fouling scaling is hydrophilic, there is a high tendency for the membrane to have pore wetting problems. The membrane scaling however has no obvious influence on the permeate flux because the evaporation area at the feed side did not decrease significantly. In all cases, the solute rejections are still promising, recording >98%. Nevertheless, more research is still needed to examine the long-term effect of crystallized salts (within membrane matrix) on separation performance.
 |
| | Fig. 8 SEM images of the (i) outer surface and (ii) cross-section of the composite membrane after testing with 50 ppm ARI solution containing (a) 0.1 M and (b) 1.0 M NaCl. | |
 |
| | Fig. 9 EDX analysis results of the membranes after testing with different salt concentrations, (a) 0.1 M and (b) 1.0 M NaCl. | |
3.4 Effect of feed temperature and feed flow rate on DCMD performance
Fig. 10 shows the effect of feed temperature on the permeate flux of PVDF–Cloisite 15A hollow fiber composite membrane in the feed solution composed of 50 ppm AR1 and 1 M NaCl. As expected, an exponential relation between permeate flux and feed temperature was observed. This trend could be explained by the Antoine equation which predicts an exponential relationship between the vapor pressure difference and temperature.15,33 At low feed temperature, heat is likely to be wasted through conduction across both the membrane material and the gas-filled membrane pores, rather than to be used for water evaporation.41 However, when the feed temperature is further increased, the latent heat of water evaporization is the main contribution to the total heat transfer which results in significant improvement in permeate flux as evidenced in this work. Although heat loss through conduction still occurs at high feed temperature, the impact can be minimized by introducing higher partial vapor pressure at the feed side.42
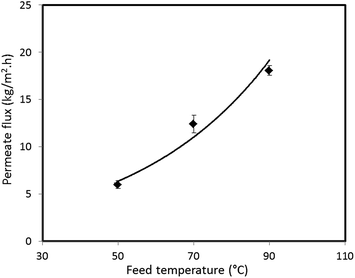 |
| | Fig. 10 Permeate flux as a function of feed temperature (conditions = hot stream: 50–90 °C at flow rate of 0.023 m s−1, cold stream: 20 °C at flow rate of 0.010 m s−1). | |
Fig. 11 shows the permeate flux of PVDF–Cloisite 15A composite membrane as a function of feed flow rate using feed solution composed of 50 ppm AR1 and 1 M NaCl. It is noticed that the effect of feed flow rate is less significant compared to feed temperature in enhancing permeate flux of membrane. The permeate flux was reported to increase by only 25% even though the feed flow rate was greatly increased by more than 100%. The slight enhancement of permeate flux at higher flow rate can be attributed to the decrease in the temperature polarization effect between the membrane surface and the bulk streams.16,43,44 Although the flux increases with increasing feed flow rate, it is important to ensure that the hydraulic pressure is lower than the wetting pressure while controlling the feed flow rate. This precaution is needed in order to prevent wetting problem during MD process.
 |
| | Fig. 11 Permeate flux as a function of feed flow rate (conditions = hot stream: 70 °C at flow rate of 0.010–0.023 m s−1, cold stream: 20 °C at flow rate of 0.010 m s−1). | |
3.5 Performance comparison of membrane distillation
Table 4 compares the performance of the self-made membrane studied in this work with previously published works for the treatment of dyeing solutions. Although other factors such as MD configuration, operating temperatures and feed properties might also affect the MD performance, in addition to the membrane property itself, the results shown in this work have revealed that the performance of the self-made PVDF–Cloisite 15A hollow fiber composite membrane is comparable or even better than the commercial membranes for textile wastewater treatment process. The results proved the in-house made membrane can be a reliable material for the DCMD process, particularly in treating effluents containing dyes and salts. Although VMD in general shows higher flux than DCMD, the objective of using DCMD in this study is due to its relatively simple operation mode and low maintenance cost. For DCMD configuration, both evaporation and condensation processes can occur simultaneously within the membrane module and it requires no external vacuum pump. Thus, it is more cost-effective to implement.
Table 4 Comparison of the membrane performance obtained in this study with the literature in the MD process for the treatment of dyeing solutions
| Membrane material |
Membrane configuration |
MD configuration |
Type of dye (concentration) |
Jv (kg m−2 h−1) |
aTf (°C) |
bTp (°C) |
cRcolor (%) |
References |
| Tf = feed temperature. Tp = permeate temperature. Rcolor = color rejection. |
| PP (commercial membrane module) |
Hollow fiber |
DCMD |
Blue E-G (5000 ppm) |
1.62 |
50 |
35 |
100 |
45 |
| PP (Enka Microdyn, USA) |
Capillary |
VMD |
MB (18.5 ppm) |
6.3 |
70 |
N/A |
100 |
15 |
| PP (Membrana GmbH, Germany) |
Capillary |
VMD |
Blue R (50 ppm) |
57 |
60 |
N/A |
>90 |
16 |
| PP (Membrana GmbH, Germany) |
Capillary |
Hybrid photocatalysis–DCMD |
AR18 (30 ppm) |
3.5 × 10−3 |
65 |
20 |
100 |
17 |
| PP (Hangzhou Kaijie membrane Company, China) |
Hollow fiber |
SPMDR |
RB5 (400 ppm) |
4.56 |
65 |
— |
100 |
18 |
| PVDF (fabricated) |
Hollow fiber |
DCMD |
RB5 (50 ppm) |
5.64 ± 0.10 |
80 |
20 |
99.83 ± 0.01 |
19 |
| PVDF (fabricated) |
Hollow fiber |
DCMD |
RB5 (500 ppm) |
9.82 ± 0.52 |
60 |
20 |
99.86 ± 0.04 |
20 |
| PVDF–Cloisite 15A (fabricated) |
Hollow fiber |
DCMD |
RB5 (50 ppm) |
10.13 ± 0.18 |
70 |
20 |
99.98 ± 0.01 |
21 |
| PVDF–Cloisite 15A (fabricated) |
Hollow fiber |
DCMD |
AR1 (50 ppm) and NaCl (1 M) |
12.42 ± 0.93 |
70 |
20 |
99.92 ± 0.07 |
This study |
4 Conclusion
In the present work, the application of DCMD using PVDF–Cloisite 15A hollow fiber composite membrane for dye and salt removal was investigated systematically. Results showed that the in-house made composite membrane demonstrated excellent results in eliminating almost all dye components (except CV dye) with consistent permeate flux recorded irrespective of dye properties. Since CV dye has high affinity towards PVDF-based membrane material and exhibits lowest MW, it can be easily absorbed to membrane matrix, altering membrane color and affecting permeate quality. With respect to dye concentration, it is found that both permeate flux and solute rejection was slightly affected with increasing dye concentration in the feed solution. Reduced permeate flux as a result of increased dye concentration can be attributed to the membrane fouling which increases mass transport resistance. Although salt concentration has no significant effect on membrane performance, the presence of crystallized salts within membrane matrix when the membrane was subject to high concentration of salt solution might need to further analyze, in particular on its effect on membrane long-term performance. In terms of process conditions, it is reported that the increase in both feed temperature and feed flow rate could enhance membrane permeate flux owing to higher latent heat of water evaporation and higher heat transfer coefficient. While this work has focused exclusively on the separation of dyes and salts in aqueous solutions, the effect of surfactants on the activity of the dyes–salts aqueous solution and the performance of membrane is recommended for future work to better examine the potential of MD for industrial wastewater. Besides, effect of solution pH and co-existing ions are also necessary to take into account to better model the textile effluent.
Acknowledgements
The authors gratefully acknowledge Universiti Teknologi Malaysia (UTM) for funding this project under Research University Grant Scheme (Tier 1) (Vot no.: Q.J130000.2509.05H48, Title: Separation and Purification of Textile Wastewater using Low-Energy Direct Contact Membrane Distillation (DCMD) Process).
References
- W. J. Lau and A. F. Ismail, Polymeric nanofiltration membranes for textile dye wastewater treatment: preparation, performance evaluation, transport modelling, and fouling control—a review, Desalination, 2009, 245, 321–348 CrossRef CAS PubMed.
- M. Marcucci, I. Ciabatti, A. Matteucci and G. Vernaglione, Membrane technologies applied to textile wastewater treatment, Ann. N. Y. Acad. Sci., 2003, 984, 53–64 CrossRef CAS PubMed.
- R. G. Saratale, G. D. Saratale, J. S. Chang and S. P. Govindwar, Bacterial decolorization and degradation of azo dyes: a review, J. Taiwan Inst. Chem. Eng., 2011, 42, 138–157 CrossRef CAS PubMed.
- B. R. Babu, A. K. Parande, S. Raghu and T. P. Kumar, Cotton Textile Processing: Waste Generation and Effluent Treatment, J. Cotton Sci., 2007, 153, 141–153 Search PubMed.
- A. Ajmal, I. Majeed, R. N. Malik, H. Idriss and M. A. Nadeem, Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview, RSC Adv., 2014, 4, 37003–37026 RSC.
- S. Sen and G. N. Demirer, Anaerobic Treatment of Synthetic Textile Wastewater Containing a Reactive Azo Dye, J. Environ. Eng., 2003, 129, 595–601 CrossRef CAS.
- M. Lucas and J. Peres, Decolorization of the Azo Dye Reactive Black 5 by Fenton and Photo-Fenton Oxidation, Dyes Pigm., 2006, 71, 236–244 CrossRef CAS PubMed.
- S. Thomas, R. Sreekanth, V. A. Sijumon, U. K. Aravind and C. T. Aravindakumar, Oxidative Degradation of Acid Red 1 in Aqueous Medium, Chem. Eng. J., 2014, 244, 473–482 CrossRef CAS PubMed.
- G. Crini, Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer, Dyes Pigm., 2008, 77, 415–426 CrossRef CAS PubMed.
- N. Ravindran and G. Balasubramani, Role of White Rot Fungi in Textile Dye Degradation, Int. J. Adv. Interdiscip. Res., 2014, 1, 38–44 Search PubMed.
- J. Chen, Q. Wang, Z. Hua and G. Du, Research and application of biotechnology in textile industries in China, Enzyme Microb. Technol., 2007, 40, 1651–1655 CrossRef CAS PubMed.
- M. Chhabra, S. Mishra and T. R. Sreekrishnan, Combination of chemical and enzymatic treatment for efficient decolorization/degradation of textile effluent: High operational stability of the continuous process, Biochem. Eng. J., 2015, 93, 17–24 CrossRef CAS PubMed.
- N. Tüfekci, N. Sivri and İ. Toroz, Pollutants of Textile Industry Wastewater and Assessment of its Discharge Limits by Water Quality Standards, Turk. J. Fish. Aquat. Sci., 2007, 7, 97–103 Search PubMed.
- J. Dasgupta, J. Sikder, S. Chakraborty, S. Curcio and E. Drioli, Remediation of textile effluents by membrane based treatment techniques: a state of the art review, J. Environ. Manage., 2015, 147, 55–72 CrossRef CAS PubMed.
- F. Banat, S. Al-Asheh and M. Qtaishat, Treatment of waters colored with methylene blue dye by vacuum membrane distillation, Desalination, 2005, 174, 87–96 CrossRef CAS PubMed.
- A. Criscuoli, J. Zhong, A. Figoli, M. C. Carnevale, R. Huang and E. Drioli, Treatment of dye solutions by vacuum membrane distillation, Water Res., 2008, 42, 5031–5037 CrossRef CAS PubMed.
- S. Mozia, A. W. Morawski, M. Toyoda and T. Tsumura, Integration of photocatalysis and membrane distillation for removal of mono- and poly-azo dyes from water, Desalination, 2010, 250, 666–672 CrossRef CAS PubMed.
- D. Qu, Z. Qiang, S. Xiao, Q. Liu, Y. Lei and T. Zhou, Degradation of Reactive black 5 in a submerged photocatalytic membrane distillation reactor with microwave electrodeless lamps as light source, Sep. Purif. Technol., 2014, 122, 54–59 CrossRef CAS PubMed.
- N. M. Mokhtar, W. J. Lau and A. F. Ismail, The potential of membrane distillation in recovering water from hot dyeing solution, Journal of Water Process Engineering, 2014, 2, 71–78 CrossRef PubMed.
- N. M. Mokhtar, W. J. Lau, B. C. Ng, A. F. Ismail and D. Veerasamy, Preparation and characterization of PVDF membranes incorporated with different additives for dyeing solution treatment using membrane distillation, Desalin. Water Treat., 2014 DOI:10.1080/19443994.2014.959063.
- N. M. Mokhtar, W. J. Lau, A. F. Ismail and B. C. Ng, Physicochemical study of polyvinylidene fluoride–Cloisite15A® composite membranes for membrane distillation application, RSC Adv., 2014, 4, 63367–63379 RSC.
- N. M. Mokhtar, W. J. Lau and A. F. Ismail, Dye wastewater treatment by direct contact membrane distillation using polyvinylidene fluoride hollow fiber membranes, J. Polym. Eng., 2014 DOI:10.1515/polyeng-2014-0214.
- P. J. Lin, M. C. Yang, Y. L. Li and J. H. Chen, Prevention of surfactant wetting with agarose hydrogel layer for direct contact membrane distillation used in dyeing wastewater treatment, J. Membr. Sci., 2014, 475, 511–520 CrossRef PubMed.
- M. S. El-Bourawi, Z. Ding, R. Ma and M. Khayet, A framework for better understanding membrane distillation separation process, J. Membr. Sci., 2006, 285, 4–29 CrossRef CAS PubMed.
- K. W. Lawson and D. R. Lloyd, Membrane distillation, J. Membr. Sci., 1997, 124, 1–25 CrossRef CAS.
- K. Hunger, Industrial Dyes, Wiley-VCH Verlag GmbH & Co, Germany, 2002 Search PubMed.
- N. A. A. Sani, W. J. Lau and A. F. Ismail, Influence of polymer concentration in casting solution and solvent–solute–membrane interactions on performance of polyphenylsulfone (PPSU) nanofiltration membrane in alcohol solvents, J. Polym. Eng., 2014, 34, 489–500 CAS.
- S. Darvishmanesh, J. Vanneste, E. Tocci, J. Jansen, F. Tasseli, J. Degreve, E. Drioli and B. Van der Bruggen, Physicochemical Characterization of Solute Retention in Solvent Resistant Nanofiltration: The Effect of Solute Size, Polarity, Dipole Moment and Solubility Parameter, J. Phys. Chem. B, 2011, 115, 14507–14517 CrossRef CAS PubMed.
- E. Alventosa-deLara, S. Barredo-Damas, E. Zuriaga-Agustí, M. I. Alcaina-Miranda and M. I. Iborra-Clar, Ultrafiltration ceramic membrane performance during the treatment of model solutions containing dye and salt, Sep. Purif. Technol., 2014, 129, 96–105 CrossRef CAS PubMed.
- E. Zuriaga-Agustí, E. Alventosa-deLara, S. Barredo-Damas, M. I. Alcaina-Miranda, M. I. Iborra-Clar and J. A. Mendoza-Roca, Performance of ceramic ultrafiltration membranes and fouling behavior of a dye–polysaccharide binary system, Water Res., 2014, 54, 199–210 CrossRef PubMed.
- A. F. Ismail and W. J. Lau, Influence of feed conditions on the rejection of salt and dye in aqueous solution by different characteristics of hollow fiber nanofiltration membranes, Desalin. Water Treat., 2009, 6, 281–288 CrossRef CAS.
- X. Wei, X. Kong, C. Sun and J. Chen, Characterization and application of a thin-film composite nanofiltration hollow fiber membrane for dye desalination and concentration, Chem. Eng. J., 2013, 223, 172–182 CrossRef CAS PubMed.
- M. Khayet and T. Matsuura, Membrane Distillation: Principles and Applications, Elsevier Science, Amsterdarm, 2011 Search PubMed.
- P. Gharbani, S. M. Tabatabaii and A. Mehrizad, Removal of Congo red from textile wastewater by ozonation, Int. J. Environ. Sci. Technol., 2008, 5, 495–500 CrossRef CAS.
- N. H. Ince and G. Tezcanh, Treatability of textile dye-bath effluents by advanced oxidation: preparation for reuse, Water Sci. Technol., 1999, 40, 183–190 CrossRef CAS.
- H. Liu and J. Wang, Treatment of radioactive wastewater using direct contact membrane distillation, J. Hazard. Mater., 2013, 261, 307–315 CrossRef CAS PubMed.
- S. Yarlagadda, V. G. Gude, L. M. Camacho, S. Pinappu and S. Deng, Potable water recovery from As, U, and F contaminated ground waters by direct contact membrane distillation process, J. Hazard. Mater., 2011, 192, 1388–1394 CrossRef CAS PubMed.
- A. M. Gamal, S. A. A. Farha, H. B. Sallam, G. E. A. Mahmoud and L. F. M. Ismail, Kinetic Study and Equilibrium Isotherm Analysis of Reactive Dyes Adsorption onto Cotton Fiber, Nat. Sci., 2010, 8, 95–110 Search PubMed.
- L. Ji, Y. Zhang, E. Liu, Y. Zhang and C. Xiao, Separation behavior of NF membrane for dye/salt mixtures, Desalin. Water Treat., 2013, 51, 3721–3727 CrossRef CAS.
- M. Gryta, Alkaline scaling in the membrane distillation process, Desalination, 2008, 228, 128–134 CrossRef CAS PubMed.
- K. Y. Wang, S. W. Foo and T. S. Chung, Mixed Matrix PVDF Hollow Fiber Membranes with Nanoscale Pores for Desalination through Direct Contact Membrane Distillation, Ind. Eng. Chem. Res., 2009, 48, 4474–4483 CrossRef CAS.
- H. Susanto, Towards practical implementations of membrane distillation, Chemical Engineering and Processing: Process Intensification, 2011, 50, 139–150 CrossRef CAS PubMed.
- S. Bonyadi and T. S. Chung, Highly porous and macrovoid-free PVDF hollow fiber membranes for membrane distillation by a solvent-dope solution co-extrusion approach, J. Membr. Sci., 2009, 331, 66–74 CrossRef CAS PubMed.
- T. C. Chen, C. D. Ho and H. M. Yeh, Theoretical modeling and experimental analysis of direct contact membrane distillation, J. Membr. Sci., 2009, 330, 279–287 CrossRef CAS PubMed.
- V. Calabro, E. Drioli and F. Matera, Membrane distillation in the textile wastewater treatment, Desalination, 1991, 83, 209–224 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)