Synthesis and adsorption property of hydrophilic–hydrophobic macroporous crosslinked poly(methyl acryloyl diethylenetriamine)/poly(divinylbenzene) (PMADETA/PDVB) interpenetrating polymer networks (IPNs)
Hebing
Li
,
Zhenyu
Fu
,
Li
Yang
,
Chong
Yan
,
Limiao
Chen
,
Jianhan
Huang
* and
You-Nian
Liu
College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, China. E-mail: jianhanhuang@csu.edu.cn
First published on 6th March 2015
Abstract
Hydrophilic–hydrophobic macroporous crosslinked poly(methyl acryloyl diethylenetriamine)/poly(divinylbenzene) (PMADETA/PDVB) interpenetrating polymer networks (IPNs) were prepared, characterized and evaluated for adsorption of salicylic acid from aqueous solution. PMADETA/PDVB IPNs were prepared by filling PDVB networks in the pores of PGMA networks according to IPNs technology and an amination reaction of PGMA/PDVB IPNs with diethylenetriamine (DETA). The structure of PMADETA/PDVB IPNs was characterized by Fourier transform infrared spectroscopy (FT-IR), N2 adsorption–desorption isotherms and chemical analysis. PMADETA/PDVB IPNs had a very large equilibrium adsorption capacity to salicylic acid and the equilibrium adsorption capacity was measured to be 196.4 mg g−1 at an equilibrium concentration of 100 mg L−1. The Freundlich model was more appropriate for fitting the equilibrium data than the Langmuir model and the adsorption was shown to be an exothermic and spontaneous process. The adsorption reached equilibrium within 200 min and the pseudo-second-order rate equation characterized the kinetic data better than the pseudo-first-order rate equation. At an initial concentration of 1035 mg L−1 and a flow rate of 84 mL h−1, the breakthrough and saturated capacities were 75.02 and 159.53 mg mL−1 wet resin, respectively, and the resin column could be completely regenerated and repeatedly by 0.01 mol L−1 of NaOH (w/v) and 20% of ethanol (v/v).
1. Introduction
Salicylic acid is a popular ingredient in many over-the-counter products. It is frequently found in lotions and creams, cleansers, medicated treatment pads and solutions. In particular, salicylic acid is widely used as an important pharmaceutical intermediate for production of medicine (aspirin), and it can be applied for the preservation of food, preservatives of glue and brighteners of cosmetics, hence the production amount of salicylic acid is very large.1,2 However, salicylic acid can cause stinging, burning, skin irritation and serious environmental problems.3–5 Even if with a low concentration of 10−4 M, it will affect the natural growth of plants.6 Recently, wastewater containing salicylic acid has been identified as a water pollutant for its ecotoxicity in water, which originates mainly from manufacturing activities of salicylic acid.7 Generally, the pharmaceutical concentrations of salicylic acid from the effluents were in the range of 10–1000 ng L−1, the detected concentration of salicylic acid was about 2098 ng L−1 in the river of Chu Chiang Delta region in China.8 As a result, efficient removal of salicylic acid from aqueous solution has received many attentions in recent years.A lot of methods and technologies including catalysis, membrane separation, oxidation, extraction and adsorption have been developed for removal of aromatic compounds,9–15 among which adsorption is proved to the most simple and efficient method.16–18 With respect to activated carbon, synthetic resins are increasingly employed for efficient removal and recovery of aromatic compounds from wastewater due to their stable physicochemical structure, diverse chemical structure, controllable pore structure and feasible regeneration property, and development of novel synthetic resins aimed at the molecular structure of the adsorbate remains a high priority and has attracted many interests in recent years.19–21 In consideration of its molecular structure, salicylic acid has a hydrophobic benzene ring as well as hydrophilic phenolic hydroxyl and carboxyl groups. Moreover, there exists an intramolecular hydrogen bonding between the phenolic hydroxyl group and the carboxyl group, which constitutes a planar hexatomic ring. Moreover, the newly formed planar hexatomic ring is relatively hydrophobic, making salicylic acid a well-balanced molecule with both of hydrophobic portion and hydrophilic portion. In 1970s, interpenetrating polymer networks (IPNs) were developed as a kind of novel polymeric materials for adsorption, and one of the important characteristics of IPNs is the force compatibility effect.22–24 The chains of one polymer networks in the IPNs are physically tangled with another chains of the other polymer networks.25–27 In addition to mutual physical entanglements, not any new chemical bond is formed between the two polymer networks in the IPNs. The strong phase separation liability will present between the hydrophobic and the hydrophilic polymer networks, and hence the hydrophobicity or the hydrophilicity of the two polymer networks composed of the IPNs is similar.28–30 Moreover, it is a great challenge to prepare typical IPNs which contains both hydrophobic and hydrophilic polymer networks, and we have obtained hydrophobic–hydrophilic IPNs composed of polydivinylbenzene (PDVB) and polyacryl diethylenetriamine (PADETA) by filling polymethylacrylate (PMA) networks in the pores of PDVB networks according to a IPNs technology and a further amination reaction of PDVB/PMA IPNs,17,18,31 and it is proposed that the PDVB/PADETA IPNs is typical IPNs with both of hydrophobic networks and hydrophilic networks. They possess superior swelling and adsorption properties in hydrophobic–hydrophilic solvent (like benzyl alcohol) and the adsorbate (salicylic acid and anthranilic acid).
In this study, we developed a novel hydrophilic–hydrophobic IPNs composed of hydrophilic PMADETA and hydrophobic PDVB and used this novel IPNs for adsorption of salicylic acid from aqueous solution. For this purpose, firstly hydrophobic–hydrophobic macroporous crosslinked poly(glycidyl methacrylate)/poly(divinylbenzene) (PGMA/PDVB) IPNs was prepared by a typical IPNs technology. Then the first hydrophobic PGMA networks were transformed to the hydrophilic poly(methyl acryloyl diethylenetriamine) (PMADETA) networks by an amination reaction, and hence the macroporous crosslinked hydrophilic–hydrophobic PMADETA/PDVB IPNs were achieved. After characterization of PMADETA/PDVB IPNs by Fourier transform infrared (FT-IR) spectroscopy, N2 adsorption–desorption isotherms and other chemical analysis, the adsorption performance of PMADETA/PDVB IPNs toward salicylic acid were investigated in detail.
2. Experimental
2.1 Materials
GMA and DVB were used as the monomer in the polymerization, and they were firstly washed by 5% of NaOH (w/v) for three times and followed by de-ionized water. After the washing process, GMA and DVB were dried by anhydrous calcium chloride and kept in the refrigerator for 24 h before use. Benzoyl peroxide (BPO) employed as the initiator in the polymerization was refined by methanol before use. Salicylic acid, toluene, n-heptane, triallyisocyanurate (TAIC) and diethylenetriamine (DETA) were all analytical reagents and used without further purification.2.2 Preparation of macroporous crosslinked PMADETA/PDVB IPNs
As shown in Scheme 1, macroporous crosslinked PMADETA/PDVB IPNs were prepared from macroporous crosslinked PGMA/PDVB IPNs by an amination reaction with DETA and the PGMA/PDVB IPNs was obtained by interpenetration of PDVB networks in PGMA networks. The macroporous crosslinked PGMA polymeric beads were prepared by a typical suspension polymerization of GMA and TAIC using toluene and n-heptane as the porogens. Toluene and n-heptane were 200% relative to the monomers (w/w) and the mass ratio between toluene and n-heptane was defined as 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The obtained PGMA beads were swollen in the mixtures of DVB, BPO, toluene and n-heptane for 24 h and the mass ratio of PGMA to DVB was determined as 1
1. The obtained PGMA beads were swollen in the mixtures of DVB, BPO, toluene and n-heptane for 24 h and the mass ratio of PGMA to DVB was determined as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The swollen PGMA beads were filtered and added into 0.05% of polyvinyl alcohol (PVA) aqueous solution (w/v). At a moderate stirring speed (190 rpm), the temperature of the reaction mixture was risen to 358 K and kept at this temperature for 12 h. The resultant PGMA/PDVB IPNs were chemically transformed to macroporous crosslinked PMADETA/PDVB IPNs by an amination reaction of PGMA/PDVB IPNs with DETA at 393 K for 15 h.
1. The swollen PGMA beads were filtered and added into 0.05% of polyvinyl alcohol (PVA) aqueous solution (w/v). At a moderate stirring speed (190 rpm), the temperature of the reaction mixture was risen to 358 K and kept at this temperature for 12 h. The resultant PGMA/PDVB IPNs were chemically transformed to macroporous crosslinked PMADETA/PDVB IPNs by an amination reaction of PGMA/PDVB IPNs with DETA at 393 K for 15 h.
2.3 Characterization
FT-IR spectra of the resins were recorded on a Nicolet 510P Fourier transform infrared instrument in 500–4000 cm−1 with a resolution of 1.0 cm−1. The Brunauer–Emmett–Teller (BET) surface area, pore volume and pore diameter distribution of the resins were determined by N2 adsorption–desorption isotherms at 77 K using a Micromeritics Tristar 3000 surface area and porosity analyzer. The weak basic exchange capacity of the resins was measured according to the ref. 32.2.4 Equilibrium and kinetic adsorption
The resins (about 0.1000 g) were mixed with 50 mL of salicylic acid at different initial concentrations. The initial concentrations of salicylic acid were pre-set to be 200.5, 401.0, 601.5, 802.0 and 1002.5 mg L−1, respectively. The mixtures were then continuously shaken at a desired temperature (298, 308 and 318 K, respectively) for 8 h until equilibrium. To determine the equilibrium concentration of salicylic acid in aqueous solution, the working curve of the standard salicylic acid solution with different known concentrations was firstly measured by UV absorbency at a wavelength of 296.5 nm on a UV 2450 spectrophotometer, and a well fitted regression equation, A = 0.02528C + 0.0096, was obtained with a correlation coefficient R2 of 0.9997. The absorbency of the residual salicylic acid solution adsorbed by the resin was then measured and the equilibrium concentration of salicylic acid, Ce (mg L−1), was calculated based on the working curve, and the equilibrium adsorption capacity of salicylic acid on the resins were calculated by conducting a mass balance of salicylic acid on the resins before and after the equilibrium. The kinetic curves for the adsorption of salicylic acid on the resins were similar to the equilibrium adsorption except that the adsorption capacity was determined in real time until equilibrium. The initial concentration of salicylic acid was pre-set to be 613.5 mg L−1 and the temperature was 298 K, respectively.2.5 Dynamic adsorption and desorption
The resins were fully immersed in de-ionized water for 24 h and 8.6 mL (1 BV) wet resins were packed densely in a glass column to assemble a resin column. The salicylic acid at an initial concentration of 1035 mg L−1 was passed through the resin column at a fixed flow rate of 9.8 BV h−1 and the residual concentration of salicylic acid from the effluent was dynamically recorded until it reached the initial concentration. 0.01 mol L−1 of NaOH (w/v) and 20% of ethanol (v/v) was applied as the desorption solvent for the dynamic desorption. At a flow rate of 3.5 BV h−1, the concentration of salicylic acid from the effluent was tested until it was about zero.3. Results and discussion
3.1 Characterization of macroporous crosslinked PMADETA/PDVB IPNs
As shown in Table 1, after interpenetration of PDVB networks in PGMA networks, the particle size of the polymer remains to be 0.4–0.6 mm, while the BET surface area and pore volume increase from 64.00 m2 g−1 and 0.3587 cm3 g−1 (PGMA) to 271.3 m2 g−1 and 0.7249 cm3 g−1 (PGMA/PDVB IPNs), respectively, which may be from the fact that the pores of the PGMA networks are supported and filled by the PDVB networks. After the amination reaction of PGMA/PDVB IPNs with DETA, the BET surface area and pore volume further increase to 290.3 cm3 g−1 and 0.8780 cm3 g−1, which may be resulted from the crosslinking of DETA between the PGMA chains.| PGMA | PMADETA | PGMA/PDVB IPNs | PMADETA/PDVB IPNs | |
|---|---|---|---|---|
| BET surface area/(cm2 g−1) | 64.00 | 39.63 | 271.3 | 290.3 |
| Pore volume/(cm3 g−1) | 0.3587 | 0.2456 | 0.7249 | 0.8780 |
| Particle size/(mm) | 0.4–0.6 | 0.4–0.6 | 0.4–0.6 | 0.4–0.6 |
| Average pore diameter/(nm) | 12.06 | 11.11 | 8.896 | 10.32 |
| Weak exchange capacity/(mmol g−1) | — | 2.898 | — | 3.479 |
Fig. 1 displays the FT-IR spectra of PGMA, PDVB, PMADETA, PGMA/PDVB IPNs and PMADETA/PDVB IPNs, respectively. It indicates that the FT-IR spectrum of PGMA/PDVB IPNs is completely superimposed by that of PGMA and PDVB. The strong vibrational band with frequency at 1728 cm−1,33 which can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the ester carbonyl groups of PGMA, is emerged in the corresponding FT-IR spectrum of PGMA/PDVB IPNs. Additionally, the strong absorption bands at 1600, 1496 and 1448 cm−1, which are related to the C
O stretching of the ester carbonyl groups of PGMA, is emerged in the corresponding FT-IR spectrum of PGMA/PDVB IPNs. Additionally, the strong absorption bands at 1600, 1496 and 1448 cm−1, which are related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of benzene ring of PDVB,34,35 are also observed in the FT-IR spectrum of PGMA/PDVB IPNs. These results reveal that, in addition to mutual physical entanglements between the PGMA and PDVB networks, not any new chemical bonds are formed and the obtained polymer complex PGMA/PDVB is typical IPNs. After the amination of PGMA/PDVB IPNs with DETA, the strong vibrational band at 1728 cm−1 is sharply weakened, whereas a new moderate band appears at 1651 cm−1 in the FT-IR spectrum of PMADETA/PDVB IPNs, and this strong band may be concerned with the C
C stretching of benzene ring of PDVB,34,35 are also observed in the FT-IR spectrum of PGMA/PDVB IPNs. These results reveal that, in addition to mutual physical entanglements between the PGMA and PDVB networks, not any new chemical bonds are formed and the obtained polymer complex PGMA/PDVB is typical IPNs. After the amination of PGMA/PDVB IPNs with DETA, the strong vibrational band at 1728 cm−1 is sharply weakened, whereas a new moderate band appears at 1651 cm−1 in the FT-IR spectrum of PMADETA/PDVB IPNs, and this strong band may be concerned with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the amide carbonyl groups.33 Additionally, another new broad and strong vibration with frequency at 3429 cm−1 is also appeared in the FT-IR spectrum of PMADETA/PDVB IPNs, and this vibration may be assigned to the N–H stretching of the –NH–/–NH2 groups or the O–H stretching of the hydroxyl groups.36–39 Moreover, these related vibrational bands of the amide carbonyl groups appear in the FT-IR spectrum of PMADETA. The weak basic exchange capacity of PMADETA/PDVB IPNs was measured to be 3.479 mmol g−1 (Table 1), whereas those of PGMA and PGMA/PDVB IPNs were determined to be 0, which suggested that the PGMA networks of PGMA/PDVB IPNs were transformed to PMADETA networks and macroporous crosslinked PMADETA/PDVB IPNs was synthesized successfully.
O stretching of the amide carbonyl groups.33 Additionally, another new broad and strong vibration with frequency at 3429 cm−1 is also appeared in the FT-IR spectrum of PMADETA/PDVB IPNs, and this vibration may be assigned to the N–H stretching of the –NH–/–NH2 groups or the O–H stretching of the hydroxyl groups.36–39 Moreover, these related vibrational bands of the amide carbonyl groups appear in the FT-IR spectrum of PMADETA. The weak basic exchange capacity of PMADETA/PDVB IPNs was measured to be 3.479 mmol g−1 (Table 1), whereas those of PGMA and PGMA/PDVB IPNs were determined to be 0, which suggested that the PGMA networks of PGMA/PDVB IPNs were transformed to PMADETA networks and macroporous crosslinked PMADETA/PDVB IPNs was synthesized successfully.
3.2 Equilibrium adsorption
PMADETA/PDVB IPNs contain hydrophilic PMADETA networks as well as hydrophobic PDVB networks in the polymer chains, which leads it to be both hydrophilic and hydrophobic, and it should have a relatively high adsorption affinity to the adsorbate with both of hydrophilic and hydrophobic portion. As a result, it is proposed that the adsorption of salicylic acid on PMADETA/PDVB IPNs should be very efficient as compared with the hydrophilic PMADETA as well as the hydrophobic PDVB.The equilibrium adsorption isotherms of salicylic acid on PMADETA/PDVB IPNs were measured in comparison with PGMA, PGMA/PDVB IPNs as well as PMADETA and the results are displayed in Fig. 2. The equilibrium adsorption capacity of salicylic acid on PMADETA/PDVB IPNs was measured to be 232.4 mg g−1, much larger than PGMA, PGMA/PDVB IPNs and PMADETA. As compared the equilibrium adsorption capacity of salicylic acid on synthetic resins with that on activated carbon, it was found that the equilibrium adsorption capacity of salicylic on synthetic resins was shown to be relatively smaller than that on activated carbon which was reported to be 351.0 mg g (ref. 40) comparative with the crosslinked resins reported in ref. 17 (230.7 mg g−1), ref. 31 (215.0 mg g−1) and ref. 41 (85.1 mg g−1). However, activated carbon can not be used repeatedly, and the strength of activated carbon was weakened after using, which is much inferior to synthetic resins. In addition, PMADETA/PDVB IPNs possessed a greater BET surface area than PGMA and PGMA/PDVB IPNs, inducing a larger equilibrium adsorption capacity. In fact, the great superiority was perfectly embodied for the adsorption of salicylic acid on PMADETA/PDVB IPNs due to the perfect polarity matching between PMADETA/PDVB IPNs and salicylic acid. The hydrophilic PMADETA networks were inclined to approach the hydrophilic portion of salicylic acid (the phenolic hydroxyl and carboxyl groups) by static interaction or hydrogen bonding,42–45 the hydrophobic PDVB networks had a relative strong adsorption affinity to the hydrophobic portion of salicylic acid (the benzene ring and the newly formed hexatomic ring between the phenolic hydroxyl and carboxyl groups) via hydrophobic interaction or π–π stacking.46–49 Therefore, PMADETA/PDVB IPNs held a highly efficient adsorption to salicylic acid.
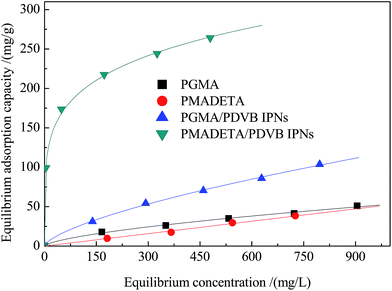 | ||
| Fig. 2 Equilibrium isotherms of salicylic acid on PGMA, PMADETA, PGMA/PDVB IPNs and PMADETA/PDVB IPNs from aqueous solution with the temperature at 298 K. | ||
Fig. 3 is the equilibrium adsorption isotherms of salicylic acid on PMADETA/PDVB IPNs from aqueous solution with the temperature at 298, 308 and 318 K, respectively. It can be observed that the equilibrium adsorption capacity of salicylic acid PMADETA/PDVB IPNs increased with increasing of the equilibrium concentration, the temperature was unfavorable for the adsorption and a higher temperature induced a lower equilibrium adsorption capacity. Langmuir and Freundlich models were frequently adopted to describe the equilibrium adsorption process,50,51 they can be arranged as:
 | (1) |
| Freundlich model: qe = KFCe1/n | (2) |
 | ||
| Fig. 3 Equilibrium isotherms of salicylic acid on PMADETA/PDVB IPNs from aqueous solution with the temperature at 298, 308 and 318 K, respectively. | ||
Both of the Langmuir and Freundlich models were applied for characterization of the equilibrium data and the corresponding characteristic parameters, qm, KL, KF and n were summarized in Table 2. Table 2 indicated that the Freundlich model was more appropriate for characterizing the equilibrium data than the Langmuir model due to the much higher correlation coefficients (R2 > 0.99). In addition, the n values for the adsorption was greater than 1, implying that the adsorption was a favorable process. The KF values decreased with increasing of the temperature, suggesting that the adsorption affinity of salicylic acid on PMADETA/PDVB IPNs was less at a higher temperature.
| Langmuir model | Freundlich model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| q m/(mg g−1) | Standard error | K L/(L mg−1) | Standard error | R 2 | K F/([(mg g−1) (L mg−1)1/n]) | Standard error | n | Standard error | R 2 | |
| 298 K | 232.4 | 14.31 | 0.2808 | 0.1400 | 0.9299 | 87.08 | 1.449 | 5.631 | 0.0980 | 0.9997 |
| 308 K | 232.4 | 14.50 | 0.1209 | 0.0566 | 0.9375 | 73.71 | 1.706 | 4.992 | 0.1058 | 0.9995 |
| 318 K | 238.3 | 13.20 | 0.0674 | 0.0248 | 0.9585 | 66.32 | 3.622 | 4.623 | 0.2131 | 0.9977 |
As the equilibrium data could be correlated by the Freundlich model, the thermodynamic parameters for the adsorption can be obtained according to eqn (3)–(5) as follows:47,52
 | (3) |
| ΔG = −nRT | (4) |
 | (5) |
All the thermodynamic parameters such as ΔH, ΔG and ΔS were calculated and the results were summarized in Table 3. In addition, the ΔH of PMADETA/PDVB IPNs was calculated to be −53.11 kJ mol−1 with the temperature at 298 K and the value is larger than some other resins reported in ref. 17 (−11.80 kJ mol−1) and ref. 31 (−14.60 kJ mol−1).
| q e/(mg g−1) | ΔH/(kJ mol−1) | Errors | ΔG/(kJ mol−1) | ΔS/(J mol−1 K−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 298 K | Errors | 308 K | Errors | 318 K | Errors | 298 K | Errors | 308 K | Error | 318 K | Errors | |||
| 80 | −53.11 | ±0.5 | −13.95 | ±0.3 | −12.78 | ±0.3 | −12.22 | ±0.3 | −131.3 | ±1.5 | −130.9 | ±1.5 | −128.5 | ±1.5 |
| 120 | −36.97 | ±0.5 | −13.95 | ±0.3 | −12.78 | ±0.3 | −12.22 | ±0.3 | −77.22 | ±1.5 | −78.51 | ±1.5 | −77.80 | ±1.5 |
| 160 | −25.51 | ±0.5 | −13.95 | ±0.3 | −12.78 | ±0.3 | −12.22 | ±0.3 | −38.79 | ±1.5 | −41.32 | ±1.5 | −41.78 | ±1.5 |
| 200 | −16.63 | ±0.5 | −13.95 | ±0.3 | −12.78 | ±0.3 | −12.22 | ±0.3 | −8.970 | ±1.5 | −12.47 | ±1.5 | −13.85 | ±1.5 |
3.3 Kinetic adsorption
Fig. 4(a) displays the kinetic curves for the adsorption of salicylic acid on PGMA, PGMA/PDVB IPNs and PMADETA/PDVB IPNs from aqueous solution, and the initial concentration of salicylic acid was set to be 613.5 mg L−1 and the temperature was 298 K, respectively. It was found that the adsorption capacity of salicylic acid on the three resins increased rapidly with increasing of the adsorption time, and the adsorption reached over 85% of the equilibrium in one hour. All of the adsorption could reach equilibrium within 200 minutes, suggesting that the adsorption was a fast process. Moreover, the adsorption on PGMA could reach equilibrium in one hour, while 120 minutes were required for the adsorption on PGMA/PDVB IPNs, 180 minutes were necessary for the adsorption on PMADETA/PDVB IPNs, suggesting that the diffusion rate of salicylic acid in the pores of PMADETA/PDVB IPNs was much slower than those of PGMA and PGMA/PDVB IPNs.Pseudo-first-order and pseudo-second-order rate equations were applied for fitting the kinetic data.53,54 They can be given as:
Pseudo-first-order rate equation: ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t | (6) |
| Pseudo-second-order rate equation: t/qt = 1/(k2qe2) + t/qe | (7) |
Table 4 summarizes the corresponding parameters according to the pseudo-first-order and pseudo-second-order rate equations. The adsorption on PGMA could only be fitted by the pseudo-second-order rate equation while those on PGMA/PDVB IPNs and PMADETA/PDVB IPNs could be fitted by both of the two equations due to the high correlation coefficients (R2 > 0.98). In particular, the k2 values on PGMA, PGMA/PDVB IPNs and PMADETA/PDVB IPNs were measured to be 0.0066, 0.0011, 0.0003 g (mg−1 min−1), accordant with the observed experimental fact that the adsorption on PGMA was the fastest while that on PMADETA/PDVB IPNs was the slowest.
| Pseudo-first-order | Pseudo-second-order | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| q m/(mg g−1) | Standard error | k 1/(min−1) | Standard error | R 2 | q m/(mg g−1) | Standard error | k 2/(g (mg−1 min−1)) | Standard error | R 2 | |
| PGMA | 29.11 | 0.4649 | 0.1293 | 0.0107 | 0.9724 | 31.21 | 0.2525 | 0.0066 | 3.769 × 10−4 | 0.9952 |
| PGMA/PDVB IPNs | 52.36 | 0.3252 | 0.0484 | 0.0011 | 0.9974 | 58.85 | 1.041 | 0.0011 | 9.710 × 10−5 | 0.9875 |
| PMADETA/PDVB IPNs | 137.7 | 1.517 | 0.0385 | 0.0016 | 0.9895 | 155.9 | 0.7732 | 0.0003 | 7.927 × 10−6 | 0.9988 |
In the adsorption of aromatic compounds on porous resin, the intra-particle diffusion process is frequently the rate-limiting step.55 The intra-particle diffusion model can be expressed as:
| qt = kpt0.5 | (8) |
By employing the intra-particle diffusion model to fit the kinetic data and Fig. 4(b) is the plotting of the qt versus t0.5. The plotting showed similar characters having two linear segments followed by a plateau. In the first stage, plotting of qt versus t0.5 gived a straight line and the straight line passes through the origin, which suggested that the intra-particle diffusion was the only rate-limited step. In particular, the kp on PGMA, PGMA/PDVB IPNs and PMADETA/PDVB IPNs were linearly fitted to be 6.35, 7.02 and 17.01, respectively, which was different from the average k2 values in Table 4.
3.4 Dynamic adsorption and desorption
Fig. 5 displays the dynamic curves for the adsorption and desorption of salicylic acid on PMADETA/PDVB IPNs resin column. Here C/C0 = 0.05 (where C is the concentration of salicylic acid from the effluent, mg L−1) is defined as the breakthrough point and the volume of the effluent to reach this point is identified as Vb. The volume of the effluent to reach the saturated point is identified as Vs and C/C0 = 0.95 is defined as the saturated adsorption point. Fig. 5 indicates that Vb and Vs were measured to be 72.42 and 154.0 BV at an initial concentration of 1035 mg L−1 and a flow rate of 9.8 BV h−1, and the corresponding adsorption capacities the breakthrough point and the saturated adsorption point could be calculated to be 75.02 and 159.53 mg mL−1 wet resin, respectively. | ||
| Fig. 5 Dynamic adsorption and desorption curves of salicylic acid on PMADETA/PDVB IPNs resin column from aqueous solution. | ||
After the dynamic adsorption, different solvents were selected for the desorption process, and the desorption ratios of PMADETA/PDVB IPNs resin column by different solvents are displayed in Fig. 6(a). It is evident that water can hardly desorb salicylic acid from the resin column and only 29.71% of salicylic acid was desorbed from the resin column as water was applied as the desorption solvent. Meanwhile, NaOH aqueous solution was effective for desorption of salicylic acid from the resin column and 96.2% of salicylic acid could be recovered by 0.01 mol L−1 of NaOH (w/v). Moreover, adding some quantities of ethanol in 0.01 mol L−1 of NaOH (w/v) could favorably increase the desorption ratio, and a mixed desorption solvent containing 0.01 mol L−1 of NaOH (w/v) and 20% of ethanol (v/v) could completely desorb salicylic acid from the resin column, and the desorption ratio reached 99.6%. Hence, 0.01 mol L−1 of NaOH (w/v) and 20% of ethanol (v/v) was applied as the desorption solvent in the dynamic desorption process. At a flow rate of 3.5 BV h−1, only 27.0 BV of the desorption solvent was shown to be enough for complete regeneration of the resin column, and the dynamic desorption capacity was calculated to be 820.6 mg, which was excellently coincident with the dynamic capacity (826.9 mg). PMADETA/PDVB IPNs were repeatedly used for five cycles of continuous adsorption–desorption process, and the result indicated that PMADETA/PDVB IPNs exhibit good reusability with remarkable regeneration behaviors (Fig. 6(b)).
4. Conclusions
We synthesized macroporous crosslinked PMADETA/PDVB IPNs by filling PDVB networks in the pores of PGMA networks and following by an amination reaction of the obtained macroporous crosslinked PGMA/PDVB IPNs with DETA. The synthesized PMADETA/PDVB IPNs was both hydrophilic and hydrophobic, which led a very large equilibrium adsorption capacity to salicylic acid containing hydrophilic and hydrophobic portion. The equilibrium adsorption capacity of salicylic acid on PMADETA/PDVB IPNs was 196.4 mg g−1 at an equilibrium concentration of 100 mg L−1 and 298 K, the Freundlich model was more appropriate for fitting the equilibrium data than the Langmuir model and the thermodynamic parameters were all negative. The pseudo-second-order rate equation was more suitable for characterizing the kinetic data than the pseudo-first-order rate equation and the adsorption in the first process could be fitted by the intra-particle diffusion model. The breakthrough and saturated dynamic adsorption capacities were measured to be 75.02 and 159.53 mg mL−1 wet resin at an initial concentration of 1035 mg L−1 and a flow rate of 9.8 BV h−1, and the resin column could be completely regenerated by 0.01 mol L−1 of NaOH (w/v) and 20% of ethanol (v/v).Acknowledgements
The National Natural Science Foundation of China (no. 21174163, 21376275 and 21446016) and South Wisdom Valley Innovative Research Team Program are gratefully acknowledged for the financial support.References
- A. Judefeind, P. J. van Rensburg, S. Langelaar and J. du Plessis, Stable isotope dilution analysis of salicylic acid and hydroquinone in human skin samples by gas chromatography with mass spectrometric detection, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 852, 300–307 CrossRef CAS PubMed.
- M. T. Jafari, Z. Badihi and E. Jazan, A new approach to determine salicylic acid in human urine and blood plasma based on negative electrospray ion mobility spectrometry after selective separation using a molecular imprinted polymer, Talanta, 2012, 99, 520–526 CrossRef CAS PubMed.
- N. Misra and P. Saxena, Effect of salicylic acid on proline metabolism in lentil grown under salinity stress, Plant Sci., 2009, 177, 181–189 CrossRef CAS.
- J. Araña, E. P. Melián, L. V. M. Rodríguez, A. A. Peña, R. J. M. Doña, D. O. González and P. J. Pérez, Photocatalytic degradation of phenol and phenolic compounds: Part I. Adsorption and FTIR study, J. Hazard. Mater., 2007, 146, 520–528 CrossRef PubMed.
- M. J. Meng, Y. H. Feng, M. Zhang, Y. Liu, Y. J. Ji, J. Wang, Y. L. Wu and Y. S. Yan, Highly efficient adsorption of salicylic acid from aqueous solution by wollastonite-based imprinted adsorbent: a fixed-bed column study, Chem. Eng. J., 2013, 225, 331–339 CrossRef CAS.
- Q. Hayata, S. Hayata, M. Irfana and A. Ahmad, Effect of exogenous salicylic acid under changing environment: a review, Environ. Exp. Bot., 2010, 68, 14–25 CrossRef.
- L. S. María, M. Andrés, D. Herminia and C. P. Juan, Recovery, concentration and purification of phenolic compounds by adsorption: a review, J. Food Eng., 2011, 105, 1–27 CrossRef.
- M. Farré, I. Ferrer, A. Ginebreda, M. Figueras, L. Olivella, L. Tirapu, M. Vilanova and D. Barceló, Determination of drugs in surface water and wastewater samples by liquid chromatography-mass spectrometry: methods and preliminary results including toxicity studies with Vibrio fischeri, J. Chromatogr. A, 2001, 938, 187–197 CrossRef.
- E. Díaz, J. I. Jiménez and J. Nogales, Aerobic degradation of aromatic compounds, Curr. Opin. Biotechnol., 2013, 24, 431–442 CrossRef PubMed.
- M. D. Marsolek, M. J. Kirisits, K. A. Gray and B. E. Rittmann, Coupled photocatalytic-biodegradation of 2,4,5-trichlorophenol: effects of photolytic and photocatalytic effluent composition on bioreactor process performance, community diversity, and resistance and resilience to perturbation, Water Res., 2014, 50, 59–69 CrossRef CAS PubMed.
- P. Veverka and K. Jeřábek, Mechanism of hypercrosslinking of chloromethylated styrene-divinylbenzene copolymers, React. Funct. Polym., 1999, 41, 21–25 CrossRef CAS.
- C. L. Zhang, L. Wu, D. Q. Cai, C. Y. Zhang, N. Wang, J. Zhang and Z. Y. Wu, Adsorption of polycyclic aromatic hydrocarbons (fluoranthene and anthracenemethanol) by functional graphene oxide and removal by pH and temperature-sensitive coagulation, ACS Appl. Mater. Interfaces, 2013, 5, 4783–4790 CAS.
- E. V. Lau, S. Gan, H. K. Ng and P. E. Poh, Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies, Environ. Pollut., 2014, 184, 640–649 CrossRef CAS PubMed.
- I. Urruzola, L. Serrano, R. Llano-Ponte, A. M. Angeles and J. Labidi, Obtaining of eucalyptus microfibrils for adsorption of aromatic compounds in aqueous solution, Chem. Eng. J., 2013, 229, 42–49 CrossRef CAS.
- X. Y. Jin and J. H. Huang, Adsorption of vanillin by an anisole-modified hyper-cross-linked polystyrene resin from aqueous solution: equilibrium, kinetics, and dynamics, Adv. Polym. Technol., 2013, 32, 221–230 CrossRef.
- D. D. Do, Adsorption analysis, World Scientific, 1998 Search PubMed.
- J. H. Huang, L. Yang, X. M. Wang, H. B. Li, L. M. Chen and Y. N. Liu, A novel post-cross-linked polystyrene/polyacryldiethylenetriamine (PST_pc/PADETA) interpenetrating polymer networks (IPNs) and its adsorption towards salicylic acid from aqueous solutions, Chem. Eng. J., 2014, 248, 216–222 CrossRef CAS.
- X. M. Wang, L. M. Chen, Y. N. Liu and J. H. Huang, Macroporous crosslinked polydivinylbenzene/polyacryldiethylenetriamine (PDVB/PADETA) interpenetrating polymer networks (IPNs) and their efficient adsorption to o-aminobenzoic acid from aqueous solutions, J. Colloid Interf. Sci., 2014, 429, 83–87 CrossRef CAS PubMed.
- B. Saha, R. J. Gill, D. G. Bailey, N. Kabay and M. Arda, Sorption of Cr(vi) from aqueous solution by Amberlite XAD-7 resin impregnated with Aliquat 336, React. Funct. Polym., 2004, 60, 223–244 CrossRef CAS.
- C. Valderrama, J. I. Barios, M. Caetano, A. Farran and J. L. Cortina, Kinetic evaluation of phenol–aniline mixtures adsorption from aqueous solutions onto activated carbon and hypercrosslinked polymeric resin (MN200), React. Funct. Polym., 2010, 70, 142–150 CrossRef CAS.
- B. Saha and M. Streat, Adsorption of trace heavy metals: application of surface complexation theory to a macroporous polymer and a weakly acidic ion-exchange resin, Ind. Eng. Chem. Res., 2005, 44, 8671–8681 CrossRef CAS.
- D. Klempner, L. H. Sperling and L. A. Utracki, Interpenetrating polymer networks, An American Chemical Society Publication, 1994 Search PubMed.
- L. H. Sperling, Interpenetrating Polymer Networks and Related Materials, Plenum, New York, 1981 Search PubMed.
- M. Tsumura, K. Ando, J. Kotani, M. Hiraishi and T. Iwahara, Silicon-based interpenetrating polymer networks (IPNs): Synthesis and properties, Macromolecules, 1998, 31, 2716–2723 CrossRef CAS.
- J. Zhang and N. A. Peppas, Synthesis and characterization of pH-and temperature-sensitive poly (methacrylic acid)/poly (N-isopropylacrylamide) interpenetrating polymeric networks, Macromolecules, 2000, 33, 102–107 CrossRef CAS.
- Y. Chang, S. F. Chen, Q. M. Yu, Z. Zhang, M. Bernards and S. Y. Jiang, Development of biocompatible interpenetrating polymer networks containing a sulfobetaine-based polymer and a segmented polyurethane for protein resistance, Biomacromolecules, 2007, 8, 122–127 CrossRef CAS PubMed.
- R. A. Stile and K. E. Healy, Poly(N-isopropylacrylamide)-based semi-interpenetrating polymer networks for tissue engineering applications. 1. effects of linear poly(acrylic acid) chains on phase behavior, Biomacromolecules, 2002, 3, 591–600 CrossRef CAS PubMed.
- J. H. Lee and S. C. Kim, Hydrophilic–hydrophobic interpenetrating polymer networks (IPN's) synthesized under high pressure. 1. Morphology, dynamic mechanical properties, and swelling behavior of polyurethane-polystyrene IPN's, Macromolecules, 1986, 19, 644–648 CrossRef CAS.
- S. Murayama, S. Kuroda and Z. Osawa, Hydrophobic and hydrophilic interpenetrating polymer networks composed of polystyrene and poly (2-hydroxyethyl methacrylate): 1. PS-PHEMA sequential IPNs synthesized in the presence of a common solvent, Polymer, 1993, 34, 2845–2852 CrossRef CAS.
- W. W. Liao, S. Q. Gao, X. L. Xie and M. C. Xu, Macroporous crosslinked hydrophobic–hydrophilic polystyrene/polyamide interpenetrating polymer network: Synthesis, characterization, and adsorption behaviors for quercetin from aqueous solution, J. Appl. Polym. Sci., 2010, 118, 3643–3648 CrossRef CAS.
- X. M. Wang, X. L. Liang, J. H. Huang and Y. N. Liu, Hydrophobic–hydrophilic polydivinylbenzene/polyacryldiethylenetriamine interpenetrating polymer networks and its adsorption performance toward salicylic acid from aqueous solutions, AIChE J., 2014, 60, 2636–2643 CrossRef CAS.
- B. L. He and W. Q. Huang, Ion exchange and adsorptive resin, Shanghai Science and Education Press, Shanghai, 1995 Search PubMed.
- M. L. Zhang and Y. Sun, Poly(glycidyl methacrylate-divinylbenzene-triallyliso cyanurate) continuous-bed protein chromatography, J. Chromatogr. A, 2001, 912, 31–38 CrossRef CAS PubMed.
- J. T. Wang, Q. M. Hu, B. S. Zhang and Y. M. Wang, Organic Chemistry, Nankai University Press, Tianjing, 1998 Search PubMed.
- A. M. Li, Q. X. Zhang, G. C. Zhang, J. L. Chen, Z. H. Fei and F. Q. Liu, Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent, Chemosphere, 2002, 47, 981–989 CrossRef CAS PubMed.
- J. H. Huang, X. Y. Jin, J. L. Mao, B. Yuan, R. J. Deng and S. G. Deng, Synthesis, characterization, and adsorption properties of diethylenetriamine-modified hypercrosslinked resins for efficient removal of salicylic acid from aqueous solutions, J. Hazard. Mater., 2012, 217–218, 406–415 CrossRef CAS PubMed.
- J. H. Huang, X. M. Wang, P. D. Patil, J. Tang, L. M. Chen and Y. N. Liu, Synthesis, characterization and adsorption properties of amide-modified hyper-cross-linked resin, RSC Adv., 2014, 4, 41172–41178 RSC.
- X. M. Wang, P. D. Patil, C. L. He, J. H. Huang and Y. N. Liu, Acetamide-modified hyper-cross-linked resin: Synthesis, characterization and adsorption performance to phenol from aqueous solution, J. Appl. Polym. Sci., 2015, 132, 41597 Search PubMed.
- X. M. Wang, K. L. Dai, L. M. Chen, J. H. Huang and Y. N. Liu, An ethanediamine-modified hypercrosslinked polystyrene resin: Synthesis, adsorption and separation properties, Chem. Eng. J., 2014, 242, 19–26 CrossRef CAS.
- M. Otero, C. A. Grande and A. E. Rodrigues, Adsorption of salicylic acid onto polymeric adsorbents and activated charcoal, React. Funct. Polym., 2004, 60, 203–213 CrossRef CAS.
- M. Otero, M. Zabkova and A. E. Rodrigues, Comparative study of the adsorption of phenol and salicylic acid from aqueous solution onto nonionic polymeric resins, Sep. Purif. Technol., 2005, 45, 86–95 CrossRef CAS.
- X. Q. Kong, M. Shan, V. Terskikh, I. Hung, Z. H. Gan and G. Wu, Solid-state O-17 NMR of pharmaceutical compounds: salicylic acid and aspirin, J. Phys. Chem. B, 2013, 117, 9643–9654 CrossRef CAS PubMed.
- Q. W. Wang, Y. H. Yang and H. B. Gao, Hydrogen Bonding in Organic chemistry, Tianjin University Press, Tianjin, 1983 Search PubMed.
- B. K. Paul and N. Guchhait, Geometrical criteria versus quantum chemical criteria for assessment of intramolecular hydrogen bond (IMHB) interaction: a computational comparison into the effect of chlorine substitution on IMHB of salicylic acid in its lowest energy ground state conformer, Chem. Phys., 2013, 412, 58–67 CrossRef CAS.
- J. H. Huang, G. Wang and K. L. Huang, Enhanced adsorption of salicylic acid onto a β-naphthol-modified hyper-cross-linked poly(styrene-co-divinylbenzene) resin from aqueous solution, Chem. Eng. J., 2011, 168, 715–721 CrossRef CAS.
- F. Wang, B. W. Liu, P. J. J. Huang and J. W. Liu, Rationally designed nucleobase and nucleotide coordinated nanoparticles for selective DNA adsorption and detection, Anal. Chem., 2013, 85, 12144–12151 CrossRef CAS PubMed.
- W. M. Zhang, Q. Du, B. C. Pan, L. Lv, C. H. Hong, Z. M. Jiang and D. Y. Kong, Adsorption equilibrium and heat of phenol onto aminated polymeric resins from aqueous solution, Colloids Surf., A, 2009, 346, 34–38 CrossRef CAS.
- A. Hirano, T. Tanaka, Y. Urabe and H. Kataura, pH- and solute-dependent adsorption of single-wall carbon nanotubes onto hydrogels: mechanistic insights into the metal/semiconductor separation, ACS Nano, 2013, 7, 10285–10295 CrossRef CAS PubMed.
- A. Werner and H. Hasse, Experimental study and modeling of the influence of mixed electrolytes on adsorption of macromolecules on a hydrophobic resin, J. Chromatogr. A, 2013, 1315, 135–144 CrossRef CAS PubMed.
- I. Langmuir, The constitution and fundamental properties of solids and liquids. Part I. Solids, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- H. M. F. Freundlich, Über die adsorption in lösungen, Z. Phys. Chem., 1906, 57A, 385–470 Search PubMed.
- H. T. Li, M. C. Xu, Z. Q. Shi and B. L. He, Isotherm analysis of phenol adsorption on polymeric adsorbents from nonaqueous solution, J. Colloid Interface Sci., 2004, 271, 47–54 CrossRef CAS PubMed.
- S. Lagergren, About the theory of so-called adsorption of soluble substances, Kungl. Svenska vetenskapsakademien, Handlingar, 1898, 24, pp. 1–39 Search PubMed.
- Y. S. Ho and G. McKay, Sorption of dye from aqueous solution by peat, Chem. Eng. J., 1998, 70, 115–124 CrossRef CAS.
- W. J. Weber and J. C. Morris, Kinetics of adsorption on carbon from solution, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, 89, 31–60 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




