An ‘all in one’ approach for simultaneous chemotherapeutic, photothermal and magnetic hyperthermia mediated by hybrid magnetic nanoparticles†
Sivakumar Balasubramanian‡
a,
Aswathy Ravindran Girija‡a,
Yutaka Nagaokaa,
Takahiro Fukudaa,
Seiki Iwaia,
Venugopal Kizhikkilotb,
Kazunori Katoa,
Toru Maekawaa and
Sakthikumar Dasappan Nair*a
aBio Nano Electronics Research Center, Graduate School of Interdisciplinary New Science, Toyo University, Kawagoe, Saitama 350-8585, Japan. E-mail: sakthi@toyo.jp; Fax: +81-366-77-1140; Tel: +81-492-39-1636, +81-492-39-1375, +81-492-39-1640
bDepartment of Respiratory Medicines, Sooriya Hospital, Chennai, India
First published on 17th February 2015
Abstract
Nanoscience and nanotechnology utilize nanoparticles to provide several remarkable applications in biomedical fields such as bio imaging, diagnosis of several diseases, and efficient drug delivery. Multifunctional magnetic nanoparticles (MNPs) are a significant type of nanomaterials that are capable of developing therapeutic as well as diagnostic (theragnostic) applications. Multifunctional hybrid magnetic nanoparticles, as efficient theragnostic representatives, have been developed. The hybrid magnetic nanoparticles (HMNP)-Au-MNPs have been assimilated into PLGA NPs along with two potent chemotherapeutic agents (curcumin and gemcitabine). Delivering chemotherapeutics into cancer cells with multiple targeting moieties is an attractive area in cancer therapeutics. In this study, the nanoconjugates have been tailored to target the pancreatic and breast cancer cells by attaching three specific targeting ligands (folate, transferrin and AS1411 aptamer). Moreover, we report a nanoparticle-based delivery system that can deliver therapeutic cargos to the cancer cells and can exert its lethality by three distinctive modes (chemotherapeutic, photothermal and magnetic hyperthermia) either independently or by the synergistic action of drug and hyperthermia. Overall our results display an ‘all in one’ approach with the same nanoformulation for the combined destruction of cancer cells. The developed nanoformulation was highly versatile in terms of the choice of implementing lethality, depending on the cancer type.
1. Introduction
Nanoparticles wherein more than one type of nanostructure is integrated into a nanoassembly, often referred as hybrid nanoparticles, have immense potential to attain multifunctional properties. Hybrid nanoparticles, comprising two or more distinctive functional components, have garnered considerable attention in recent times.1–7 Several magnetic hybrid carbon nanotubes have been developed for the MR imaging and MR-guided photothermal treatment of tumors in recent years.8–11 Shi and coworkers demonstrated the application of another hybrid nanocomposite based on multi-walled carbon nanotubes (MWCNTs) labeled with quantum dots (QDs) that was used for efficient in vivo imaging.12 Recently, Cheng et al. developed multifunctional hybrid nanoparticles by incorporating upconversion luminescence, superparamagnetism, and optical absorption in the near-infrared (NIR) region with photostability. The hybrid NPs were utilized for multimodal imaging (optical and magnetic resonance imaging) and magnetically targeted cancer therapy, as well as emphasized the potential of the application of multifunctional hybrid nanostructures in the field of theragnostic agents for cancer.5 Multifunctional hybrid magnetic nanoparticles (HMNPs) owing to their unique advantages over mono-functional NPs have been the focus of extensive investigation. For example, gold (Au) and MNPs are two excellent candidates that exhibit distinctive properties among the different class of NPs. Owing to their intrinsic magnetic properties, MNPs (magnetite or maghemite) have immense potential in biomedical fields, including MRI, cancer therapy, magnetic targeting, drug delivery, and magnetic hyperthermia (MHT) for tumor therapy.13–15 On the other hand, Au NPs have been exploited significantly in biological applications, such as sensing and tumor therapy, and they are conjugated to target the ligands, for example DNA and proteins.16–20 In addition, Au NPs enjoy inimitable optical properties due to their strong optical absorption that alter with fluctuations in their natural environment. For their outstanding biocompatibility, Au NPs and MNPs have been thoroughly investigated for biomedical applications.21–24 Hence, the development of hybrid materials, such as MNPs for MHT and Au that holds the benefits of surface plasmon phenomena, are extremely appropriate in cancer nanotechnology.Polymer-based nanoparticles perform as ideal candidate for the delivery of various drugs, genes, vaccines, peptides, and proteins to the site of interest during the in vivo applications.25–27 Among the polymeric nanoparticles, poly(lactic-co-glycolic acid) (PLGA) is of the most commonly employed biodegradable polymers in the field of nanodrug delivery. The hydrolysis of PLGA results in lactic acid and glycolic acid that are easily metabolized by the body via the Krebs cycle and are eliminated as carbon dioxide and water. Nanoparticles based on PLGA are approved by the US FDA and European Medicine Agency (EMA) in several drug delivery systems in humans.28 Although there are several other nanodrug delivery agents, such as peptides (cell penetrating peptides), carbohydrates, and liposomes, the acceptability and applicability of PLGA nanoparticles is highly appreciated. The approval from the world wide regulatory organizations for PLA/PLGA-based nanodrug products is considered as a major reason for the extensive utilization of these biodegradable polymers over other nanoparticles.29 We used PLGA NPs for the incorporation of HMNPs and therapeutic agents to impart drug-mediated cytotoxicity. Curcumin (Cur) and Gemcitabine (Gem) were encapsulated and the therapeutic potential was investigated. The therapeutic efficiency of Cur is well established and has been used against various cancers. Apart from its therapeutic ability as an anticancerous agent Cur has been widely used for its anti-inflammatory, anti-bacterial, anti-parasitic, anti-oxidant and anti-mutagenic effects.30–32 It has been delivered in combination with other drugs for the efficient treatment of cancer. Gem is a nucleoside analog and has been successfully used against different cancers, especially pancreatic cancer and breast cancer.33–35 In our study we made the nanoconjugates specific by attaching three targeting moieties (AS1411, folate and transferrin). Cancer cells specifically overexpress several proteins and receptors relative to normal healthy cells. We targeted nucleolin, a protein that is overexpressed in pancreatic and breast cancer cells.36,37 Also, these cells are known to over expresses folate and transferrin receptors.38–40 We conjugated these ligands in order to maximize the internalization of the nanoconjugate in the cancer cells by a receptor-mediated endocytosis. The drug molecules entrapped in PLGA NPs are predominantly released in a passive manner, upon simple diffusion by hydrolysis or enzymatic digestion of the PLGA matrix. This process is quite slow and could take several days or weeks.41 However, these polymeric NPs are sensitive to physiochemical conditions, so that the active release of drugs can be achieved in a controllable manner by triggering the inherent properties of incorporated NPs. For example, an external magnetic field can be applied, if the incorporated NP is magnetite or maghemite. Similarly, NIR irradiation can also induce the release of drug, since MNPs and Au NPs are incorporated into PLGA NPs. Also, with active targeting through three different targeting ligands and the incorporation of two active drugs and Au-MNPs, the nanoconjugate can be exploited for three different modes of cancer cell damage. The first one is by the action of cytotoxic drugs. Since the NP is triple targeted, it augments the uptake of NP by the cancer cells thus resulting in the damage of cancer cells by drug-mediated cytotoxicity. The second mode is by the photothermal ablation of cancer cells by the MNP and Au components of the HMNP. The cells are pretreated with nanoconjugate and irradiated with NIR, 800 nm, which triggers the generation of heat by MNPs; moreover, owing to the surface plasmon resonance effect of Au, collectively results in the photothermal ablation of cancer cells. The third mode is by MHT, exploiting the magnetic component of HMNP. The exposure of cancer cells to the presence of an external magnetic field also triggers the release of drug from PLGA NPs and thus MHT can promote drug delivery into tumor,42 and increase the drug toxicity.
Herein, well-dispersed HMNPs of Au-MNP-PLGA have been developed and conjugated with three targeting ligands. This multifunctional nanoconjugate is designed to track the internalized NP, and integrate chemotherapy, photo-thermotherapy, and MHT simultaneously into one single system. Into this multipotential nanosystem, two highly efficient therapeutic agents were successfully loaded and a pH responsive release of drugs was observed. The antiproliferation of cancer cells mediated by the nanoconjugate and the incorporation of Au and MNPs were used to demonstrate the feasibility of using these nanocomposites in photothermal ablation and MHT mediated cancer cell ablation. Furthermore, a synergistic effect of targeted chemo-, photo- and thermo-therapy was projected to destroy cancer cells.
2. Experimental section
2.1. Materials
Ferrous chloride tetra-hydrate (FeCl2·4H2O), ferric chloride hexa-hydrate (FeCl3·6H2O), tri sodium citrate, NaOH, and ethyl acetate were procured from Kanto Chemicals, Japan. Gold III chloride trihydrate (HAuCl4·3H2O), folic acid, PLGA, and transferrin were purchased from Sigma-Aldrich, (St. Louis, MO, USA). N-(3-Dimethyl aminopropyl)-N-ethylcarbodiimidehydro-chloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Tokyo Chemical Industries, Japan. Curcumin (Cur) was gifted from AVT Spices Ltd., India. Gemcitabine (Gem), trypan blue, and 0.025% trypsin were purchased from Sigma-Aldrich. LIVE/DEAD® Viability/Cytotoxicity Assay Kit, Alamar blue toxicology kit, DAPI and Lysotracker were purchased from Invitrogen. Aptamer AS1411 (NH2-5′-(GGTGGTGGTGGTTGTGGTGGTGGTGG)-3′) was purchased from Operon.2.2. Preparation of Au-MNPs
Well-dispersed MNP that served as heterogeneous seeds were synthesized by a previously reported procedure.43,44 Briefly, 17 mM of FeCl3·6H2O and 8.5 mM of FeCl2·4H2O were dissolved in 200 mL DDW. The mixture was heated at 80 °C with continuous magnetic stirring under a nitrogen atmosphere. To this vigorously stirred and nitrogen atmosphere-protected solution, a mixture of ammonium hydroxide (1 M, 50 mL) and sodium citrate (1 M, 10 mL) were added, resulting in the pale yellow solution color shifting to brown and finally to black. The solution was allowed to stir for an additional 30 min, and the solution was then cooled to room temperature. Gold was coated onto this mixture. 0.5 mL of as-synthesized MNP was transferred into 100 mL water. Sodium citrate solution (155.2 mM, 0.35 mL) was added and this mixture solution was stirred vigorously and boiled. During boiling, the magnetite particles would be oxidized either partially or completely to maghemite before their being coated.45,46 HAuCl4 solution (10 mM, 5 mL) was injected rapidly, and the originally pale-yellow colored solution changed to brown and then to the deep red characteristic of gold colloids. The heating mantle was detached 15 min after injection, and the stirring was continued for another 15 min. After several min, dark purple NPs were obtained by separating and rinsing with the help of a magnetic field. The finally obtained dried Au-MNPs were used for further experiments.2.3. Preparation of drug loaded Au-MNP-PLGA-NPs
We further used the as synthesized Au-MNPs and therapeutic agents for encapsulation into PLGA NP by well-known solvent evaporation technique.47,48 In our study we used two drugs (a) natural drug Cur and (b) synthetic drug Gem, to explore the therapeutic prospective of nanoconjugate against cancer cells. In a typical synthesis, 200 mg of PLGA was dissolved in 2 mL of ethyl acetate solution. 10 mg of as synthesized Au-MNPs, 10 mg of Cur and 10 mg of Gem were added under magnetic stirring for 30 min. 5 mL of 5% PVA was added dropwise and ultrasonication was carried out for 2 min. The mixture was then added dropwise to 100 mL of 0.3% PVA solution and vigorous magnetic stirring was continued for another 3 h. The mixture was then centrifuged at 8000 rpm for 15 min and the pellet was washed three times with double distilled water. The finally obtained dual drug and Au-MNPs encapsulated PLGA NPs (DD-Au-MNP-PLGA-NPs) were freeze-dried and were used for further experiments. Based on solvent evaporation techniques, we have also synthesized plain PLGA-Au-MNPs, and single drug formulations (Cur-Au-MNP-PLGA-NPs, Gem-Au-MNP-PLGA-NPs) for comparative studies in cell lines.2.4. Preparation of triple targeted nanoconjugate
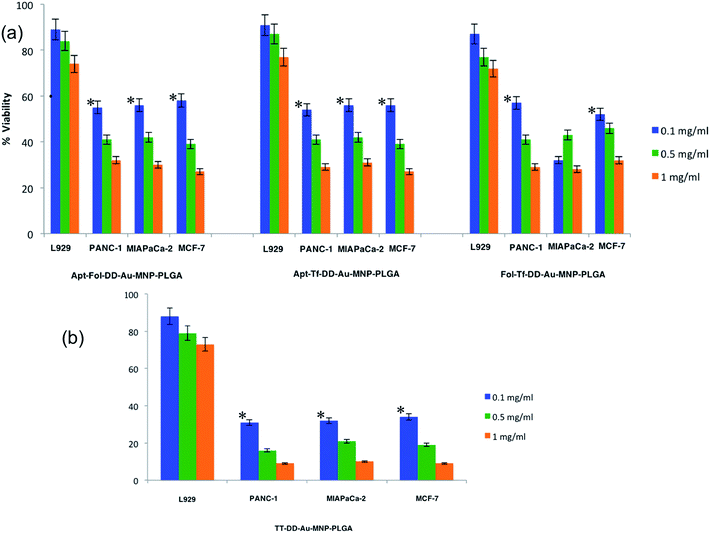
The conjugation of targeting moieties onto NPs is envisioned for specific cellular binding and internalization through the receptor-mediated endocytosis. For active cancer targeting, we chose folate, transferrin and AS1411 aptamer for conjugation onto NPs, as folate receptors, transferrin receptors and nucleolin proteins are reported to be expressed abundantly in all cancer cell lines. For the biofunctionalization of DD-Au-MNP-PLGA NPs with aptamer, folate and transferrin, approximately 1 mg mL−1 of NPs was washed three times with 250 μL aliquots of a 10 mM PBS (pH 7.4) buffer and incubated with 200 μL of 400 mM EDC and 200 μL of 100 mM NHS for 1 h at room temperature with gentle shaking. To this, a 20 μL aliquot of a 100 mM solution of AS1411 aptamer and Tf and folate (20 μL of 1 mg mL−1) was added, and mixed well, and kept under gentle shaking for 4 h at room temperature. After the incubation period, the NPs were washed three times using 20 mM Tris–HCl, 5 mM MgCl2 at pH 8.0.49,50 The resulting triple targeted DD-Au-MNP-PLGA NPs (TT-DD-Au-MNP-PLGA) were resuspended in DNase-RNase-free water and used for further analyses. Based on the same protocol, we have synthesized Apt-Fol-DD-Au-MNP-PLGA, Fol-Tf-DD-Au-MNP-PLGA and Apt-Tf-DD-Au-MNP-PLGA to compare the targeting efficiency of triple targeted dual drug loaded NP (TT-DD-Au-MNP-PLGA).2.5. Physiochemical characterization of nanoconjugates
The interior structure of as-prepared Au-MNP and Au-MNP-PLGA NPs were characterized using field emission TEM (JEOL's JEM 2200 FS TEM). EDS analysis was carried out to confirm the elemental composition of the NPs using a TEM-EDS (JEOL JED-2300T) instrument. A dispersion of these NPs was dropped onto the TEM grid, desiccated overnight, and subsequently analyzed. The surface morphology of the NPs was performed by a scanning electron microscope (JEOL's JSM 7400 SEM, JEOL, Japan). The sample for SEM was diluted by double distilled water, dropped onto the silica substrate, dried under vacuum and subsequently used for SEM analysis. The magnetization measurements of Au-MNP and TT-DD-Au-MNP-PLGA NPs were performed in a vibrating sample magnetometer (VSM Model 7407, Lakeshore cryotronics, Inc). The absorption spectra were recorded on a Beckman Coulter Life Sciences UV/Vis spectrophotometer, DU730. The conjugation of the targeting moieties to the nanoparticle was confirmed by a Kratos-Axis X-ray photoelectron spectrometer (Shimadzu, Japan). For XPS studies, the diluted samples were placed on a silica substrate, dried and characterized. Drug loading and release profiles from the nanoconjugates were recorded by UV/Vis absorption spectrophotometry. Briefly, 1 mg of TT-DD-Au-MNP-PLGA NPs was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The absorbance of the collected supernatant was checked and recorded at 430 nm for curcumin and at 268 nm for Gem. The concentration of free drug was calculated from the absorbance value, which was based on the standard curve for Cur and Gem. The in vitro drug release profile of curcumin and Gem from TT-DD-Au-MNP-PLGA NPs in PBS at different pH (pH 7.4 and pH 4.5) was performed. 10 μg of sample was dispersed into 50 mL of PBS buffer at pH 7.4 and pH 4.5. The sample was kept in a water bath shaker with a shaking speed of 120 rpm at 37 °C. At specific time intervals, 1 mL of supernatant from the sample was collected and 1 mL fresh PBS buffer was added to compensate for the loss. The absorbance of the collected sample was recorded at 430 nm for Cur and 268 nm for Gem. The in vitro drug release profile was calculated by the following formula:
000 rpm for 30 min. The absorbance of the collected supernatant was checked and recorded at 430 nm for curcumin and at 268 nm for Gem. The concentration of free drug was calculated from the absorbance value, which was based on the standard curve for Cur and Gem. The in vitro drug release profile of curcumin and Gem from TT-DD-Au-MNP-PLGA NPs in PBS at different pH (pH 7.4 and pH 4.5) was performed. 10 μg of sample was dispersed into 50 mL of PBS buffer at pH 7.4 and pH 4.5. The sample was kept in a water bath shaker with a shaking speed of 120 rpm at 37 °C. At specific time intervals, 1 mL of supernatant from the sample was collected and 1 mL fresh PBS buffer was added to compensate for the loss. The absorbance of the collected sample was recorded at 430 nm for Cur and 268 nm for Gem. The in vitro drug release profile was calculated by the following formula:| Drug release (%) = (DR/DT) × 100 |
2.6. Cell culture
Human pancreatic cell lines (PANC-1 and MIAPaCa2) were cultured in RPMI 1640 with 10% FBS, 1% L-glutamax, 1% sodium pyruvate and penicillin/streptomycin (100 units per mL) at 37 °C in a humidified 5% CO2 atmosphere until confluent. Human breast adenocarcinoma cells, MCF-7 and a control mouse fibroblast cell line (L929) were grown in Dulbecco's Modified Eagle's Medium (DMEM) with 10% heat-inactivated fetal bovine serum and penicillin/streptomycin (100 units per mL) in T25 flasks and maintained at 37 °C in a humidified 5% CO2 atmosphere. The cancer cells were subcultured every 3 days and the cells were maintained in glass-base dish for confocal studies, 6 well plates for flow cytometry, 96 well plates for cytotoxicity studies and in 35 mm plates for MHT and photothermal ablation studies.2.7. Cellular internalization of nanoconjugates
To examine the effect of the targeting moieties attached onto NPs for the effective delivery of therapeutics precisely to the desired cells, confocal analyses were performed. Cancer cells (PANC-1, MIAPaCa2 and MCF-7) and a control cell (L929) were grown on glass base dishes and incubated with TT-DD-Au-MNP-PLGA NPs for 4 h. At the end of the incubation period, the cell monolayers were rinsed three times with cold PBS buffer (0.01 M, pH 7.4) to remove excess NPs. The cells were stained with a nuclear label (DAPI) and were analyzed by a confocal microscope. Also, the cells were stained with lysotracker red to mark the location of lysosomes within the cells to understand the localization of NPs within the cell. Fresh PBS (0.01 M, pH 7.4) buffer was added to the culture plates after incubation and the cells were viewed and imaged under confocal laser microscopy.2.8. Investigation of the cytotoxicity imparted by nanoconjugates
| Relative cell viability = Asample/Acontrol × 100 |
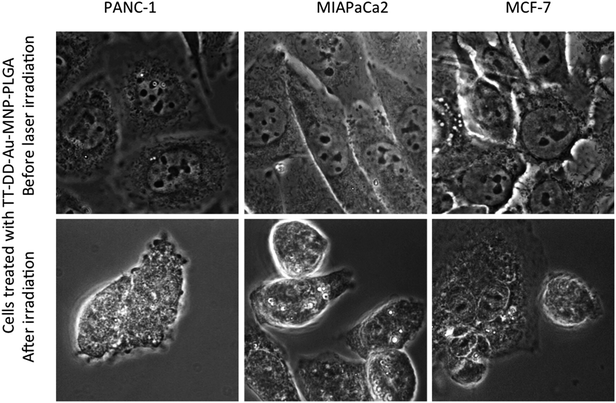
All the experiments were performed in triplicate. The cells were also plated on six-well plates to examine the therapeutic efficacy of TT-DD-Au-MNP-PLGA by phase contrast microscopy.
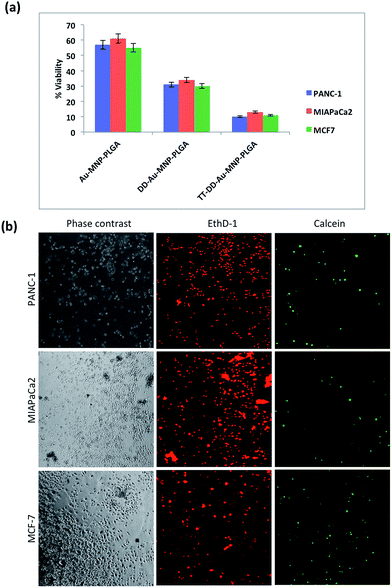
The encapsulation of Cur and Gem could lead to severe cytotoxicity by inducing apoptosis in cancer cells. The determination of the apoptotic cell population when treated with TT-DD-Au-MNP-PLGA was determined by flow cytometry and confocal microscopy with a Annexin V-FITC and a propidium iodide apoptosis kit. For flow cytometry, the cells under study were grown in 6 well plates at a density of 2.5 × 106 cells, and treated with an appropriate concentration of TT-DD-Au-MNP-PLGA. After incubation, the cells were washed with PBS and resuspended in 1× binding buffer. 5 μL of Annexin V FITC conjugate and 10 μL of propidium iodide solution were added to each cell suspension. The cells were protected from light and incubated at room temperature for 10 min, and flow cytometry was carried out. Confocal microscopy was performed for qualitative determination. Cells were grown in a glass base dish and incubated overnight at 37 °C. The next day, the medium was removed and fresh medium containing TT-DD-Au-MNP-PLGA was added. Post incubation, the cells were washed three times with PBS to remove excess NPs. Subsequently, cells were stained with Annexin V conjugate and PI based on the manufacture's protocol. The images were viewed under confocal microscopy.
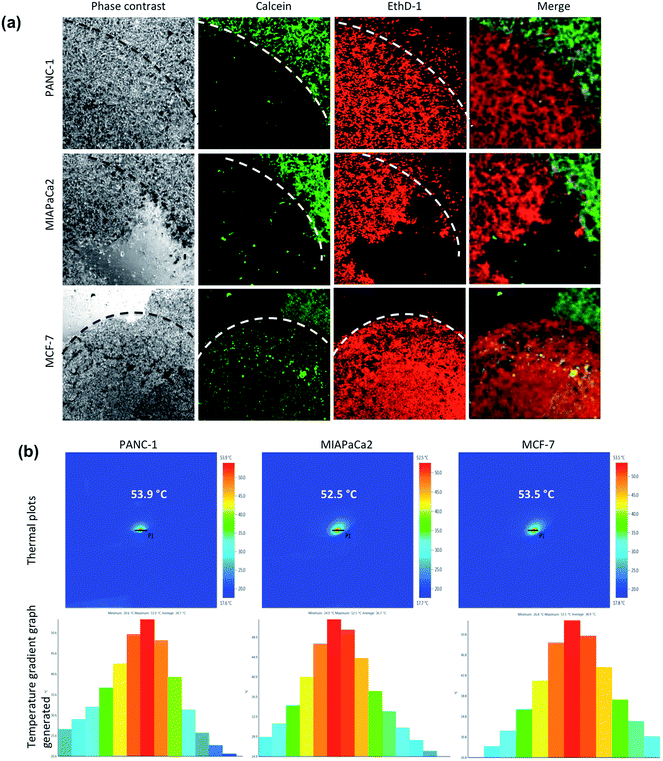
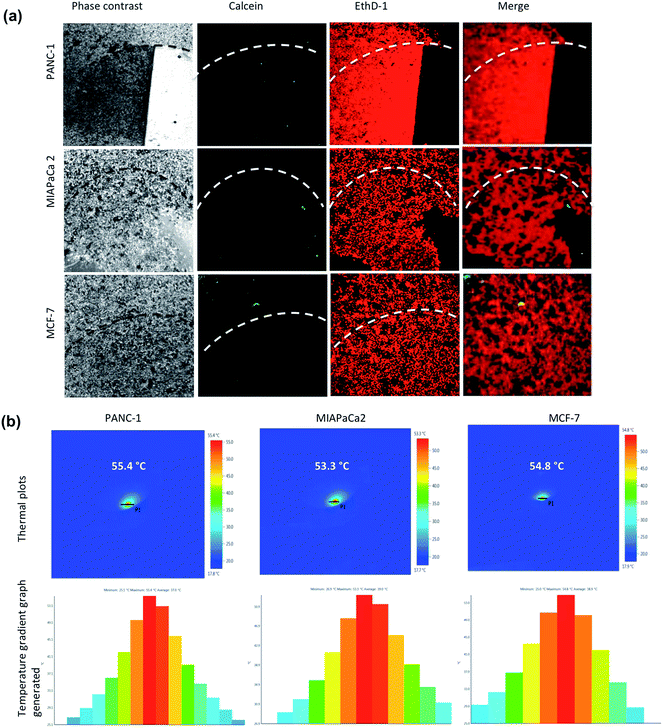
The LIVE/DEAD® Viability/Cytotoxicity Assay Kit was used for the simultaneous determination of live and dead cells after MHT on cancer cells. Briefly, cells were grown in glass base dish for 24 h with the standard medium and the cells were then replenished with fresh medium containing TT-DD-Au-MNP-PLGA. After incubation for 4 h, the unbound NPs were removed by washing and the cells were exposed to the AC magnetic field (H = 18.03 kA m−1, B = 166.25 A, frequency = 305 KHz) for 60 min. After hyperthermia, the cells were washed and then stained with the reagents in the live/dead viability kit. The images were viewed under confocal microscopy.
2.9. Statistical analysis
All quantitative results were obtained from triplicate samples. The standard deviation values are indicated as error bars in the result plots. The values were considered statistically significant when *p < 0.05.3. Results and discussion
3.1. Characterization of NPs
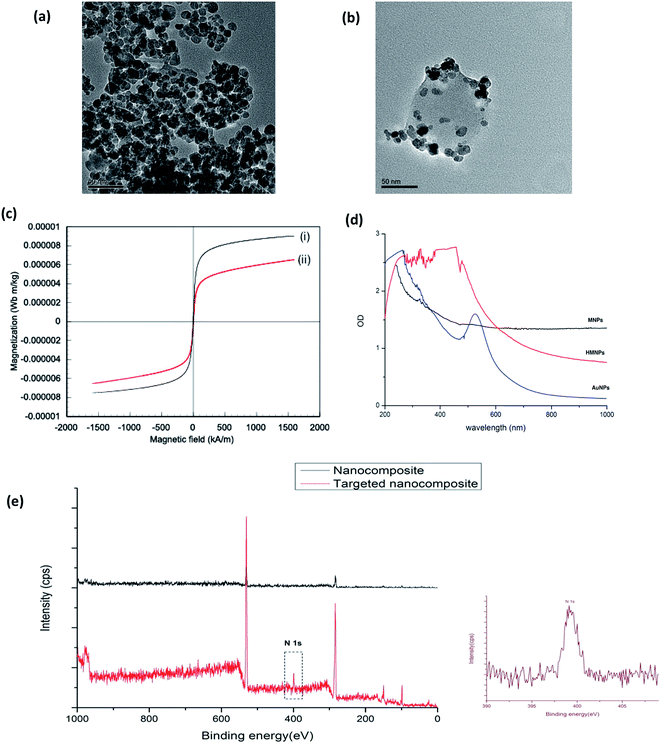
The TEM observations of the plain Au-MNPs and Au-MNPs-PLGA are shown in Fig. 1a and b. Spherical Au-MNPs with an average size in the range of 12–15 nm were observed. Au-MNPs-PLGA exhibited several MNPs encapsulated into single PLGA NPs and also several Au-MNPs were adsorbed on the surface of PLGA NPs, which was observed by the TEM analysis. The NPs revealed high monodispersity devoid of any aggregation with an average size of 100 nm. Energy-dispersive X-ray spectroscopy (EDS) analysis was done to analyze the presence of Fe, Au, C and O in the MNPs and Au-MNPs-PLGA (Fig. S1†). The SEM analysis of Au-MNPs-PLGA also supported the TEM observations of monodisperse, spherical shaped nanoparticles with an average size of 100 nm (Fig. S2†). The magnetic properties of bare Au-MNPs and TT-DD-Au-MNPs-PLGA were studied in VSM at 300 K, and the obtained results are shown in Fig. 1c. The remanent magnetization (Mr) and coercivity (Hc) were close to zero, which clearly indicates a characteristic of superparamagnetism. A slight decrease in magnetization for TT-DD-Au-MNPs-PLGA can be attributed to the PLGA coating. An external magnet could very easily affect the magnetic behavior of the Fe3O4 core in PLGA NPs and these conjugates can be influenced by an external magnetic field. The UV-Visible absorption spectra (Fig. 1d) of Au NPs shows that the Au absorbance peak from the surface plasmon absorption varies around 510–530 nm. The aqueous suspension of bare MNPs exhibits a continuous and broad absorption spectrum extending to the NIR region. It has been reported that the MNPs (iron oxides) absorb light in the range of 200–1000 nm.51,52 NIR absorption is perfect for photothermal therapy owing to its deep tissue penetration. The hybrid nanoparticles also exhibited broad absorbance in the NIR region.53–59 The attachment of targeting ligands on the hybrid NPs was confirmed by X-ray photoelectron spectroscopy (XPS). For XPS studies, we analyzed targeted and non-targeted nanocomposites and the results are presented in (Fig. 1e). In the case of targeted NPs, C and N peaks along with those from Si and O arising from the substrate were seen. The N peak in the targeted NP could possibly be from the conjugated aptamer, folate and transferrin on PLGA-MNPs, thus providing a sign of the applicable functionalization on the NPs. In the case of non-targeted NPs, the N peak was absent, demonstrating the lack of biofunctionalization. The absence of Fe and Au peaks clearly indicated that the Au-MNPs and drug were entrapped inside PLGA NPs.An efficient drug loading capability in biocompatible nanocarriers has a direct influence on the therapeutic effect of the nanosystem. We attained an encapsulation efficiency of 69% and 77% for curcumin and Gem, respectively, within the PLGA NPs. The slow, steady and sustained release of encapsulated therapeutic drugs from nanocarriers is the basic concern for the proficient development of nanoformulations in biomedical applications especially for diseases like cancer. The understanding of polymeric NPs to physiological changes, such as sensitivity to temperature, pH, light and external stimuli that triggers a sustained release of drugs is gaining greater impact.60 The in vitro release of Cur and Gem from TT-DD-Au-MNPs-PLGA was examined in PBS at physiological pH (pH 7.4) and acidic conditions related to the endosome (pH 4.5). Fig. S3† shows the in vitro release profile of Cur and Gem from NPs into PBS over a period of 144 h. A pH responsive release of drugs was observed from the PLGA NPs. As represented in Fig. S3,† the release of drugs from nanoformulations demonstrated a slow and sustained release profile over 144 h. The release kinetics at pH 4.5 and 7.4 within 144 h exhibited that the pH strongly influenced release of both drugs from NPs. We have observed 79% of curcumin release and around 90% of Gem in a period of 144 h at pH 4.5. The release of drug in acidic conditions benefits greater percentage of release of encapsulated drugs that support anticancer drug delivery.
3.2. Cellular internalization of NPs
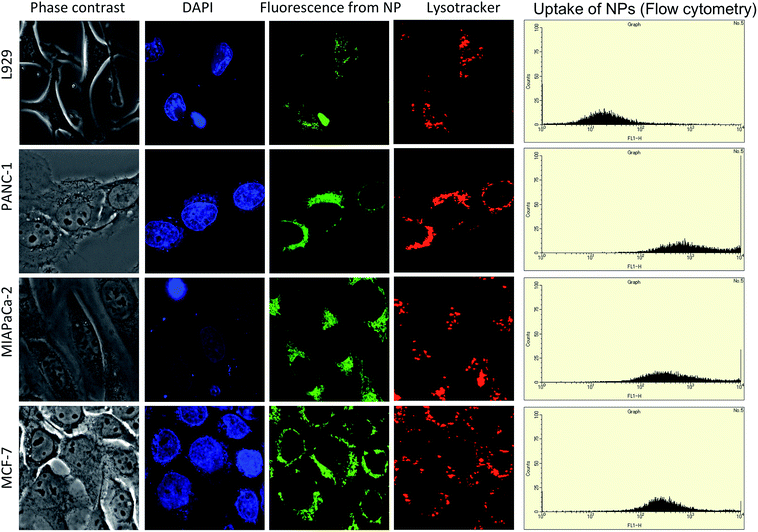
We performed confocal laser scanning microscopy to confirm the cellular uptake of single targeted NPs and TT-DD-Au-MNPs-PLGA. Since Cur was used as one of the therapeutic agents, we exploited its inherent fluorescence to track the uptake of NPs. The internalization of NPs was studied in cancer cells (PANC-1, MIAPaCa2, MCF-7) and control cells (L929). When the cancer cells were treated with triple targeted NPs, we observed huge accumulation of the same in the cell cytoplasm emphasizing the enhanced uptake compared with that of single targeted NPs (Fig S4–S6†). The flow cytometry analysis of the uptake of triple targeted NPs were substantially higher (about 10 times) in cells compared to the cells treated with single targeted NPs (Fig. 2). The results clearly demonstrated that targeting with aptamer, folate and Tf were highly specific to nucleolin, folate and Tf receptors, which were over expressed in pancreatic cancer and breast cancer cells. As presented in the Fig. 2, the images confirmed the accumulation of particles within the lysosomes, indicating the endosome-mediated entry of the same. The overlapping signal of the green fluorescence from Cur and the red fluorescence from lysosomes confirm the presence of NP in the lysosomes. In the case of L929, the low intensity of fluorescence when compared to the cancer cells demonstrates the specificity of NPs towards cancer cells.3.3. Investigation of cytotoxicity imparted by nanoconjugates
To confirm the results of cytotoxicity by TT-DD-Au-MNP-PLGA NPs, we performed apoptosis/necrosis assay using an Annexin V-PI protocol. We assume that the drugs (Cur and Gem) in the nanoconjugates trigger the apoptotic pathway in cancer cells upon treatment and enhance cell death. Just after the induction of apoptosis, phosphatidylserine (PS) is translocated from the inner plasma membrane to the outer membrane. Annexin V-FITC has a strong affinity for PS and it binds to PS, thus detecting the apoptotic cell population. The confocal microscopy images and flow cytometry results of cells treated with NPs are shown in Fig. S9.† The flow cytometry results reveal the existence of a significant portion of early apoptotic and late apoptotic cell populations following treatment with TT-DD-Au-MNP-PLGA NPs in cancer cells. We have observed 95.51%, 86.68% and 93.24% of apoptotic cells in PANC-1, MIAPaCa2 and MCF-7, respectively.
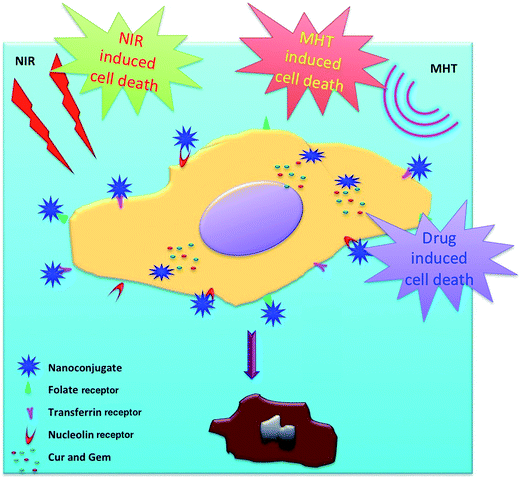
The plausible mechanism of the action of multi targeted dual drug loaded hybrid MNPs resulting in the destruction of cancer cells by trimodal lethality (drug induced, NIR irradiation and MHT) is shown schematically in Fig. 8.
4. Conclusion
In this study, we present the development of HMNP with gold and MNPs. The NPs were further incorporated into PLGA and efficiently tagged with three ligands. The therapeutic potential of the nanoconjugate was imparted by two potent chemotherapeutic agents (Cur and Gem). The HMNPs was used for combined chemo-, photo- and MHT therapy against pancreatic and breast cancer cell lines. These HMNP based nanoconjugates exhibited high drug loading capacities accompanied with a pH-sensitive release of encapsulated drugs. This in turn can reduce the nonspecific release of therapeutic agents during circulation, and augment the effectiveness of targeted delivery of anti-cancer drugs. The presence of three targeting ligands extensively boosted the uptake of the nano-conjugates by cancer cells. Cur and Gem exhibited excellent anti-proliferative effects on cancer cells. In addition to the cytotoxic effects of incorporated drugs, the exceptional photo-thermal ablation (attributed by Au and MNP) and MHT (contributed by MNPs) enhanced the ablation of cancer cells with the advantage of triple targeting. The investigated tri-modal lethality attained with the hybrid MNPs proved exceptionally proficient. Thus, we anticipate that the HMNPs will open numerous exciting prospects for the precise delivery of cargo (therapeutic agents) to cancerous tumors as well as be highly convenient for the guidance of targeted therapies in the future. All together, this ‘all in one’ nano-conjugate system is suggested as an ideal candidate for future multi-modal therapeutic strategies against cancer cells.Acknowledgements
B. Sivakumar thank the Rotary Yoneyama Foundation, Japan for providing fellowship for conducting his doctoral course. Also, part of this study has been supported by a grant for the programme of the strategic research foundation at private universities S1101017, organized by the MEXT, Japan since April 2012.References
- K. F. Leung, S. Xuan, X. Zhu, D. Wang, C. P. Chak, S. F. Lee, W. K. W. Ho and B. C. T. Chung, Chem. Soc. Rev., 2012, 41, 1911–1928 RSC.
- G. A. Sotiriou, F. Starsich, A. Dasargyri, M. C. Wurnig, F. Krumeich, A. Boss, J. C. Leroux and S. E. Pratsinis, Adv. Funct. Mater., 2014, 24, 2818–2827 CrossRef CAS.
- Y. Su, X. Wei, F. Peng, Y. Zhong, Y. Lu, S. Su, T. Xu, S. T. Lee and Y. He, Nano Lett., 2012, 12, 1845–1850 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. Li, X. Zeng, M. Shao, S. T. Lee and Z. Liu, Biomaterials, 2012, 33, 2215–2222 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. Li, J. Chen, C. Wang, M. W. Shao, S. T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 50, 7385–7390 CrossRef CAS PubMed.
- W. Zhang, Z. Y. Guo, D. Q. Huang, Z. M. Liu, X. Guo and H. Q. Zhong, Biomaterials, 2011, 32, 8555–8561 CrossRef CAS PubMed.
- M. Chu, X. Pan, D. Zhang, Q. Wu, J. Peng and W. Hai, Biomaterials, 2012, 33, 7071–7083 CrossRef CAS PubMed.
- G. Korneva, H. Ye, Y. Gogotsi, D. Halverson, G. Friedman, J. C. Bradley and K. G. Kornev, Nano Lett., 2005, 5, 879–884 CrossRef CAS PubMed.
- J. Miyawaki, M. Yudasaka, H. Imai, H. Yorimitsu, H. Isobe, E. Nakamura and S. Iijima, Adv. Mater., 2006, 18, 1010–1014 CrossRef CAS.
- D. Yang, F. Yang, J. Hu, J. Long, C. Wang, D. Fu and Q. Ni, Chem. Commun., 2009, 4447–4449 RSC.
- H. Wu, G. Liu, Y. Zhuang, D. Wu, H. Zhang, H. Yang, H. Hu and S. Yang, Biomaterials, 2011, 32, 4867–4876 CrossRef CAS PubMed.
- D. Shi, Y. Guo, Z. Dong, J. Lian, W. Wang, G. Liu, L. Wang and R. C. Ewing, Adv. Mater., 2007, 19, 4033–4037 CrossRef CAS.
- Y. M. Huh, Y. W. Jun, H. T. Song, S. Kim, J. S. Choi, J. H. Lee, S. Yoon, K. S. Kim, J. S. Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 12387–12391 CrossRef CAS PubMed.
- C. C. Berry and A. S. G. Curtis, J. Phys. D: Appl. Phys., 2003, 36, R198–R206 CrossRef CAS.
- M. Shinkai, M. Yanase, M. Suzuki, H. Honda, T. Wakabayashi, J. Yoshida and T. Kobayashi, J. Magn. Magn. Mater., 1999, 194, 176–184 CrossRef CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS PubMed.
- S. Park, K. A. Brown and K. Hamad-Schifferli, Nano Lett., 2004, 4, 1925–1929 CrossRef CAS.
- D. Zanchet, C. M. Micheel, W. J. Parak, D. Gerion and A. P. Alivisatos, Nano Lett., 2001, 1, 32–35 CrossRef CAS.
- M. E. Aubin, D. G. Morales and K. Hamad-Schifferli, Nano Lett., 2005, 5, 519–522 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nano Today, 2007, 2, 18–29 CrossRef.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- H. Liu, J. Wu, J. H. Min and Y. K. Kim, J. Alloys Compd., 2012, 537, 60–64 CrossRef CAS PubMed.
- H. Okada and H. Toguchi, Crit. Rev. Ther. Drug Carrier Syst., 1995, 12, 1–99 CrossRef CAS.
- M. L. Hans and A. M. Lowman, Curr. Opin. Solid State Mater. Sci., 2002, 6, 319–327 CrossRef CAS.
- A. Mahapatro and D. K. Singh, Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines, J. Nanobiotechnol., 2011, 9, 55 CrossRef CAS PubMed.
- F. Danhier, E. Ansorena, J. M. Silva, R. Coco, A. L. Breton and V. Préat, J. Controlled Release, 2012, 161, 505–522 CrossRef CAS PubMed.
- H. Sah, L. A. Thoma, H. R. Desu, E. Sah and G. C. Wood, Int. J. Nanomed., 2013, 8, 747–765 CrossRef PubMed.
- A. Goel, A. B. Kunnumakkara and B. B. Aggarwal, Biochem. Pharmacol., 2008, 75, 787–809 CrossRef CAS PubMed.
- A. S. Strimpakos and R. A. Sharma, Antioxid. Redox Signaling, 2008, 10, 511–545 CrossRef CAS PubMed.
- T. W. Corson and C. M. Crews, Cell, 2007, 130, 769–774 CrossRef CAS PubMed.
- H. A. Burris 3rd, M. J. Moore, J. Andersen, M. R. Green, M. L. Rothenberg, M. R. Modiano, M. C. Cripps, R. K. Portenoy, A. M. Storniolo, P. Tarassoff, R. Nelson, F. A. Dorr, C. D. Stephens and D. D. Von Hoff, J. Clin. Oncol., 1997, 15, 2403–2413 CAS.
- M. M. Di, C. R. Di, M. Macchini, E. Nobili, S. Vecchiarelli, G. Brandi and G. Biasco, Oncol. Rep., 2010, 23, 1183–1192 Search PubMed.
- C. R. Patra, R. Bhattacharya, E. Wang, A. Katarya, J. S. Lau, S. Dutta, M. Muders, S. Wang, S. A. Buhrow, S. L. Safgren, M. J. Yaszemski, J. M. Reid, M. M. Ames, P. Mukherjee and D. Mukhopadhyay, Cancer Res., 2008, 68, 1970–1978 CrossRef CAS PubMed.
- M. Derenzini, V. Sirri, D. Trere and R. L. Ochs, Lab. Invest., 1995, 73, 497–502 CAS.
- B. Sarcevic, R. Lilischkis and R. L. Sutherland, J. Biol. Chem., 1997, 272, 33327–33337 CrossRef CAS PubMed.
- J. Pan and S. S. Feng, Biomaterials, 2009, 30, 1176–1183 CrossRef CAS PubMed.
- Z. Qian, H. Li, H. Sun and K. Ho, Pharmacol. Rev., 2002, 54, 561–587 CrossRef CAS.
- A. Widera, K. J. J. Kim, E. D. Crandall and W. C. Shen, Pharm. Res., 2003, 20, 1231–1238 CrossRef CAS.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS.
- G. Kong, R. D. Braun and M. W. Dewhirst, Cancer Res., 2000, 60, 4440–4445 CAS.
- Z. L. Liu, H. B. Wang, Q. H. Lu, G. H. Du, L. Peng, Y. Q. Du, S. M. Zhang and K. L. Yao, J. Magn. Magn. Mater., 2004, 283, 258–262 CrossRef CAS PubMed.
- Q. H. Lua, K. L. Yaoa, D. Xic, Z. L. Liua, X. P. Luoc and Q. Ning, J. Magn. Magn. Mater., 2006, 301, 44–49 CrossRef PubMed.
- J. L. Lyon, D. A. Fleming, M. B. Stone, P. Schiffer and M. E. Williams, Nano Lett., 2004, 4, 719–723 CrossRef CAS.
- J. Tang, M. Myers, K. A. Bosnick and L. E. Brus, J. Phys. Chem. B, 2003, 107, 7501–7506 CrossRef CAS.
- M. S. Cartiera, E. C. Ferreira, C. Caputo, M. E. Egan, M. J. Caplan and W. M. Saltzman, Mol. Pharm., 2010, 7, 86–93 CrossRef CAS PubMed.
- S. Balasubramanian, A. R. Girija, Y. Nagaoka, S. Iwai, M. Suzuki, V. Kizhikkilot, Y. Yoshida, T. Maekawa and S. D. Nair, Int. J Nanomed., 2014, 9, 437–459 Search PubMed.
- A. Aravind, P. Jeyamohan, R. Nair, S. Veeranarayanan, Y. Nagaoka, Y. Yoshida, T. Maekawa and D. S. Kumar, Biotechnol. Bioeng., 2012, 109, 2920–2931 CrossRef CAS PubMed.
- B. Sivakumar, R. G. Aswathy, Y. Nagaoka, S. Iwai, M. Suzuki, K. Venugopal, K. Kato, Y. Yoshida, T. Maekawa and D. Sakthikumar, RSC Adv., 2013, 3, 20579–20598 RSC.
- H. E. Ghandoor, H. M. Zidan, M. M. H. Khalil and M. I. M. Ismail, Int. J. Electrochem. Sci., 2012, 7, 5734–5745 Search PubMed.
- J. Tang, M. Myers, K. A. Bosnick and L. E. Brus, J. Phys. Chem. B, 2003, 107, 7501–7506 CrossRef CAS.
- H. Chen, J. Burnett, F. Zhang, J. Zhang, H. Paholaka and D. Sun, J. Mater. Chem. B, 2014, 2, 757–765 RSC.
- S. Ghosh, S. Dutta, E. Gomes, D. Carroll, R. D'Agostino, J. Olson, M. Guthold and W. H. Gmeiner, ACS Nano, 2009, 3, 2667–2673 CrossRef CAS PubMed.
- R. Romero-Aburto, T. N. Narayanan, Y. Nagaoka, T. Hasumura, T. M. Mitcham, T. Fukuda, P. J. Cox, R. R. Bouchard, T. Maekawa, D. S. Kumar, S. V. Torti, S. A. Mani and P. M. Ajayan, Adv. Mater., 2013, 25, 5632–5637 CrossRef CAS PubMed.
- Y. Su, X. Wei, F. Peng, Y. Zhong, Y. Lu, S. Su, T. Xu, S. T. Lee and Y. He, Nano Lett., 2012, 12, 1845–1850 CrossRef CAS PubMed.
- S. Shen, F. Kong, X. Guo, L. Wu, H. Shen, M. Xie, X. Wang, Y. Jin and Y. Ge, Nanoscale, 2013, 5, 8056–8066 RSC.
- M. Chu, Y. Shao, J. Peng, X. Dai, H. Li, Q. Wu and D. Shi, Biomaterials, 2013, 34, 4078–4088 CrossRef CAS PubMed.
- H. Chen, J. Burnett, F. Zhang, J. Zhang, H. Paholak and D. Sun, J. Mater. Chem. B, 2014, 2, 757–765 RSC.
- C. Goncalves, P. Pereira and M. Gama, Materials, 2010, 3, 1420–1460 CrossRef CAS PubMed.
- J. Li and M. Gu, IEEE J. Sel. Top. Quantum Electron., 2010, 16, 989–996 CrossRef.
- M. Chu, Y. Shao, J. Peng, X. Dai, H. Li, Q. Wu and D. Shi, Biomaterials, 2013, 34, 4078–4088 CrossRef CAS PubMed.
- N. K. Prasad, K. Rathinasamy, D. Panda and D. Bahadur, J. Mater. Chem., 2007, 17, 5042–5051 RSC.
- H. L. Rodríguez-Luccioni, M. Latorre-Esteves, J. Meńdez-Vega, O. Soto, A. R. Rodríguez, C. Rinaldi and M. Torres-Lugo, Int. J. Nanomed., 2011, 6, 373–380 Search PubMed.
- L. Asín, M. R. Ibarra, A. Tres and G. F. Goya, Pharm. Res., 2012, 29, 1319–1327 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00168d |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |