Electrospun polymer nanofiber membrane electrodes and an electrolyte for highly flexible and foldable all-solid-state supercapacitors†
Abstract
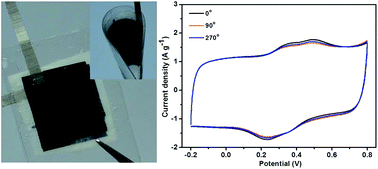
A sandwiched symmetric all-solid-state supercapacitor consisting of flexible electrospun polyaniline (PANI)/carbonized polyimide (CPI) nanocomposite membrane electrodes and a poly(vinyl alcohol) (PVA)/poly(acrylic acid) (PAA) nanofiber membrane-reinforced PVA/H3PO4 gel separator has been successfully fabricated. Needle-like PANI nanoparticles are vertically formed on the high electrically conductive three-dimensional CPI nanofiber network, resulting in an efficiently improved specific surface area of the PANI nanoparticles and faster electrolyte ion penetration and electron transfer in the active electrode material. The synergistic effect thus generates remarkably higher specific capacitance of 379 F g−1 at 0.5 A g−1 and longer cycle life with a retention of 94% at 1 A g−1 for PANI/CPI nanocomposite membrane electrodes, compared with those (209 F g−1, 56%) of neat PANI powder. Furthermore, electrospun PVA/PAA nanofiber membrane-reinforced PVA/H3PO4 gel separator exhibits superior mechanical properties over the traditional PVA/H3PO4 gel separator, thus endowing the entire device with high flexibility and foldability. Therefore, electrospinning is a very promising technique for the preparation of highly flexible and foldable electrode and separator materials with hierarchical structures in high-performance new energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...