Palladium-catalyzed oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide†
Pei Liu,
Jian Han,
Chang Peng Chen,
Da Qing Shi* and
Ying Sheng Zhao*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: yszhao@suda.edu.cn; dqshi@suda.edu.cn
First published on 17th March 2015
Abstract
A practical palladium-catalyzed γ-oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide with PhI(OAc)2 as oxidant was developed. Selective alkoxylation or acetoxylation of oxalyl amide protected benzyl amine was achieved in high yield. The oxalyl amide protected α-substituted propylamines could be transformed into acetoxylated products in good to excellent yields.
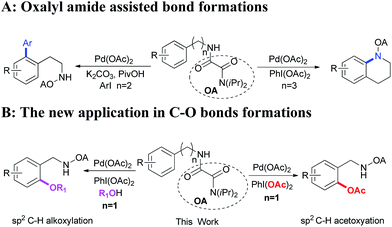
Directing-group assisted high site selective C–H functionalization has aroused considerable attention in the last decades and a significant number of transformations have emerged as powerful methods in organic synthesis.1,2 Among the reports, a variety of functional groups, such as oximes,3 pyridine,4 oxazolines,5 amides,6 carboxylic acids,7 amines,8 hydroxyls,9 esters,10 etc.11 were developed as directing groups to improve regioselectivity and reactivity in the transition-metal-catalyzed C–H functionalizations. The selective oxygenation of C–H bonds has regained much attention because of its important application in biosynthesis and post-synthetic modification.12,13 In a ground-breaking report, Sanford and co-workers developed oxime-directed acetoxylation of the C(sp3)–H bonds via palladium catalyst using PhI(OAc)2 as oxidant.14 Later, they found the pyridine was also a powerful directing group on the site selective oxygenation of C–H bonds.15 Yu and co-workers reported the palladium-catalyzed oxazoline directed stereoselective acetoxylation of methyl group by employing inexpensive t-BuOO2Me as oxidant.16 Palladium-catalyzed picolinamide-directed oxygenation of C(sp2)–H and C(sp3)–H bonds using PhI(OAc)2 as oxidant was demonstrated by Chen and co-workers in a mild condition.17 Interestingly, Shaoo and co-workers discovered the β-C(sp3)–H acetoxylation could happen at room temperature by applying S-methy-S-2-pyridylsulfoximine as the directing group.18 Recently, the β-alkoxylation of methylene even could be achieved under the assistance of 8-aminoquinlone with cyclic hypervalent iodine (I3+) as oxidant by Rao and co-workers.19 Although the significant progress has already achieved, the further development of practical oxygenation C–H bonds to synthesize useful molecules remains an important task. Herein, we report a practical protocol for palladium-catalyzed γ-oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide (Scheme 1B).
Oxalyl amide, which was made from two general reagents of diisopropylamine and oxalyl chloride through SN type reaction, was discovered by our group and emerged as a powerful directing group for amine derivatives.20 It was demonstrated that it not only had an ability to enable rarely reported C–C bond formation at δ and ε positions,20a,b but also promoted the intramolecular amination to form six-membered heteroatom rings (Scheme 1A).20c Based on previous work, we tried to prepare the four-membered azetidine with oxalyl amide protected valine. However, only acetoxylated product was observed by GC-MS. It was possible that the steric effect of diisopropylamine inhibited the intramolecular C–N formation (Scheme 2). Inspired by this result, we tried to develop a selective protocol for the palladium-catalyzed oxalyl amide-directed γ-oxygenation of amine derivatives.
At the outset of our study, we first treated the oxalyl amide protected benzyl amine 4a, PhI(OAc)2, and Pd(OAc)2 (5 mol%) in a mixture of methanol and toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) at 100 °C in a sealed tube to explore the alkoxylation reaction. It's not surprising that the product of 5a was observed in 71% yield analysed by GC (Table 1, entry 1) and the rare dialkoxylated product was observed in 24% yield. Encouraged by the preliminary result, the reaction condition was further optimized to achieve selective transformation (entry 2). The alkoxylation reaction could afford mono-alkoxylated product in 83% yield at 60 °C. The less expensive oxidants, such as K2S2O8, BQ and DDQ, were tested and failed to give any desired products, along with starting material recovered (entries 3–5, 14). Solvents screening revealed that toluene was the best solvent, providing excellent yield of 5a (entries 2, 6–9). The additives of PivOH, (BuO)2PO2H and AcOH had a slightly suppression effect on this transformation. Amino acid (Ac-Gly-OH), which was a super ligand in many transformations,21 also had the similar result as PivOH on this oxalyl amide assisted C–H oxygenation (entries 10–13). Controlling experiments confirmed that no reaction happened without palladium catalyst, indicating the irreplaceable role of Pd(OAc)2 for this transformation (entry 15).
6) at 100 °C in a sealed tube to explore the alkoxylation reaction. It's not surprising that the product of 5a was observed in 71% yield analysed by GC (Table 1, entry 1) and the rare dialkoxylated product was observed in 24% yield. Encouraged by the preliminary result, the reaction condition was further optimized to achieve selective transformation (entry 2). The alkoxylation reaction could afford mono-alkoxylated product in 83% yield at 60 °C. The less expensive oxidants, such as K2S2O8, BQ and DDQ, were tested and failed to give any desired products, along with starting material recovered (entries 3–5, 14). Solvents screening revealed that toluene was the best solvent, providing excellent yield of 5a (entries 2, 6–9). The additives of PivOH, (BuO)2PO2H and AcOH had a slightly suppression effect on this transformation. Amino acid (Ac-Gly-OH), which was a super ligand in many transformations,21 also had the similar result as PivOH on this oxalyl amide assisted C–H oxygenation (entries 10–13). Controlling experiments confirmed that no reaction happened without palladium catalyst, indicating the irreplaceable role of Pd(OAc)2 for this transformation (entry 15).
| Entrya | Oxidant | Additive | Solvent | Yield (%) |
|---|---|---|---|---|
| a 4a (0.1 mmol), MeOH (0.05 mL), Pd(OAc)2 (5 mol%), oxidant (0.15 mmol), additive (0.03 mmol), solvent (0.3 mL), in a 25 mL sealed tube; yields were based on GC using tridecane as standard.b 100 °C.c No Pd(OAc)2 was used. | ||||
| 1b | PhI(OAc)2 | — | Toluene | 71 |
| 2 | PhI(OAc)2 | — | Toluene | 90(83) |
| 3 | DDQ | — | Toluene | — |
| 4 | Cu(OAc)2 | — | Toluene | — |
| 5 | BQ | — | Toluene | — |
| 6 | PhI(OAc)2 | — | CH3OH | 44 |
| 7 | PhI(OAc)2 | — | CH3CN | 40 |
| 8 | PhI(OAc)2 | — | PhCl | 88 |
| 9 | PhI(OAc)2 | — | m-Xylene | 84 |
| 10 | PhI(OAc)2 | PivOH | Toluene | 86 |
| 11 | PhI(OAc)2 | (BuO)2PO2H | Toluene | 82 |
| 12 | PhI(OAc)2 | AcOH | Toluene | 85 |
| 13 | PhI(OAc)2 | Ac-Gly-OH | Toluene | 82 |
| 14 | K2S2O8 | — | Toluene | — |
| 15c | PhI(OAc)2 | — | Toluene | — |
Employing the optimized reaction conditions, we then explored the scope of benzyl amines, and representative data were listed in Table 2. To our great delight, a remarkable broad substrates scope of benzyl amines was achieved. The electron-donating functional groups were tolerated in this transformation, and gave the corresponding alkoxylated products in good to excellent yields (5b, 5d–5g, 5i). The electron-withdrawing groups such as F, Cl, Br were also tolerated, affording the corresponding products in good yields. The steric effect had an important influence on the regioselectivity of this transformation. As all the results were observed selectively taking place at the less hindered site (5g, 5i, 5l, 5m).
The scope of alcohols turned out to be very broad. A variety of alcohols was tolerated and gave well to excellent yields (Table 3, 5a, 5n–5u). Generally, the primary alcohols, such as ethanol, n-propanol and n-octanol, were all transformed into the corresponding ethers in good yields. It's worthy to mention that the 2-bromoethanol generated the corresponding ether in moderate yield, which was a useful synthon for further transformation (5r). Delightfully, bulk alcohol could also be employed, affording corresponding ethers in moderate yields (5s–5u). The more hindered isopropyl alcohol reacted slowly, yielding the product 5t in acceptable yield. Unfortunately, some other alcohols, such as tertiary alcohol, phenol and cyclic alcohol, were also tested and failed to give corresponding products.
Considering the reaction mechanism, the acetoxylation of amines at γ positions might be realized without the alcohol as solvent. Reaction optimization quickly, the excellent yields of acetoxylated products was observed with acetic acid (2 equiv.) as additive, using PhI(OAc)2 as oxidant in toluene under palladium catalyst. Remarkable functional groups, such as Cl–, Br–, I–, Me–, and MeO–, were well tolerated, and afforded the corresponding products in moderate to good yields. Interestingly, the substrate of 4a gave the diacetoxylated product at higher reaction temperature in moderate yield (6i). Although the role of acetic was unclear, on the basis of preliminary studies and pioneering reports, acetic acid might act as a stabilizer during the catalytic cycle (ESI†) (Table 4).
Encouraged by the success of oxalyl amide assisted oxygenation of C(sp2)–H bonds, we tried to expand the alkoxylation to unactive C(sp3)–H bonds. Gratifyingly, γ-acetoxylation of C(sp3)–H bonds can proceed slowly under the assistance of oxalyl amide with acetic acid as additive (Table 5). Interestingly, the substrate of 7f could be converted into diacetoxylated product 8f in good yield.
Inspired by the high regioselectivity of mono-alkoxylation (Tables 2 and 3), and exceptive example of 6i, we might construct the complex arene by two steps. Delightfully, sequential oxygenation was successfully achieved in total 67% yields in two steps (Scheme 3).
The synthetic utility of this new developed protocol was also examined in the gram-scale reaction. The catalyst loading could be reduced to 1 mol% and the desired product was obtained in 5.8 g. Under the basic condition, the auxiliary could be removed to furnish the potential useful product 9d (Scheme 4).
Although the mechanistic detail of this palladium-catalyzed oxalyl amide directed-oxygenation still remains uncertain, based on experimental results and pioneering works,22 we proposed a possible mechanism showed in Scheme 5. The first step involved a chelate-directed C–H activation of the substrate to generate the five-membered cyclopalladium intermediate (I) through a CMD pathway. The PdII was further oxidized to a PdIV intermediate (II) by PhI(OAc)2. When the alcohol was added, the ligand of AcO– could be replaced with alcohol to form intermediate (III). Reductive elimination afforded the oxygenated products.
In summary, we have developed a practical palladium-catalyzed γ-oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide using PhI(OAc)2 as oxidant. Alkoxylation of benzyl amine derivative could be achieved with 10–20 equivalent amount of alcohol at a mild condition. Both C(sp2)–H and C(sp3)–H bonds of oxalyl amide protected amine derivatives were converted into corresponding acetoxylated products in moderate to excellent yields. Sequential oxygenation of 4a was also successful observed in moderate yield. Gram scale reaction was examined via 1 mol% palladium catalyst affording the desired product in 5.8 g. This new developed approach is an expandation of oxalyl amide assisted C–H functionalization in synthetic chemistry.
Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of Jiangsu Province of China (L210903913), a start-up fund (Q410901212) from Soochow University and the Young National Natural Science Foundation of China (no. 21402133). The PAPD is also gratefully acknowledged.Notes and references
- (a) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (b) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (c) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (d) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (e) H. M. L. Davies, J. Du Bois and J.-Q. Yu, Chem. Soc. Rev., 2011, 40, 1855 RSC; (f) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC; (g) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2011, 45, 788 CrossRef PubMed; (h) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (i) S. R. Neufeldt and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936 CrossRef CAS PubMed; (j) J. J. Mousseau and A. B. Charette, Acc. Chem. Res., 2012, 46, 412 CrossRef PubMed; (k) J. Bariwal and E. Van der Eycken, Chem. Soc. Rev., 2013, 42, 9283 RSC; (l) M. L. Louillat and F. W. Patureau, Chem. Soc. Rev., 2014, 43, 901 RSC.
- (a) B. D. Dangel, J. A. Johnson and D. Sames, J. Am. Chem. Soc., 2001, 123, 8149 CrossRef CAS; (b) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (c) D. A. Alonso, C. Najera, I. M. Pastor and M. Yus, Chem.–Eur. J., 2010, 16, 5274 CrossRef CAS PubMed; (d) A. N. Campbell, P. B. White, I. A. Guzei and S. S. Stahl, J. Am. Chem. Soc., 2010, 132, 15116 CrossRef CAS PubMed; (e) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (f) T. R. Newhouse and P. S. Baran, Angew. Chem., 2011, 123, 3422 CrossRef; (g) P. S. Baran and T. Newhouse, Angew. Chem., Int. Ed., 2011, 50, 3362 CrossRef PubMed; (h) M. C. White, Science, 2012, 335, 807 CrossRef CAS PubMed; (i) E. M. Simmons and J. F. Hartwig, Nature, 2012, 483, 70 CrossRef CAS PubMed.
- (a) H.-Y. Thu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048 CrossRef CAS PubMed; (b) A. R. Dick and M. S. Sanford, Tetrahedron, 2006, 62, 2439 CrossRef CAS PubMed; (c) S. R. Neufeldt and M. S. Sanford, Org. Lett., 2009, 12, 532 CrossRef PubMed; (d) C.-L. Sun, N. Liu, B.-J. Li, Y. Wang and Z.-J. Shi, Org. Lett., 2009, 12, 184 CrossRef PubMed; (e) Z. Shi, D. C. Koester, M. Boultadakis-Arapinis and F. Glorius, J. Am. Chem. Soc., 2013, 135, 12204 CrossRef CAS PubMed.
- (a) A. R. Dick, J. W. Kampf and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 12790 CrossRef CAS PubMed; (b) X. Chen and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634 CrossRef CAS PubMed; (c) H.-Y. Thu, W.-Y. Yu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048 CrossRef CAS PubMed; (d) M. Tobisu, Y. Ano and N. Chatani, Chem.–Asian. J., 2008, 3, 1585 CrossRef CAS PubMed; (e) Z. Q. Lei, H. Li, Y. Li, X. S. Zhang, K. Chen, X. Wang, J. Sun and Z. J. Shi, Angew. Chem., Int. Ed., 2012, 51, 2690 CrossRef CAS PubMed; (f) K. Parthasarathy, A. R. Azcargorta, Y. Cheng and C. Bolm, Org. Lett., 2014, 16, 2538 CrossRef CAS PubMed; (g) S. Yu and X. Li, Org. Lett., 2014, 16, 1200 CrossRef CAS PubMed.
- (a) X. Chen, J.-J. Li, X.-S. Hao, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2005, 128, 78 CrossRef PubMed; (b) R. Giri, Y. Lan, P. Liu, K. N. Houk and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 14118 CrossRef CAS PubMed; (c) B. Li and P. H. Dixneuf, Chem. Soc. Rev., 2013, 42, 5744 RSC.
- (a) V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154 CrossRef CAS PubMed; (b) D.-H. Wang, X.-S. Hao, D.-F. Wu and J.-Q. Yu, Org. Lett., 2006, 8, 3387 CrossRef CAS PubMed; (c) X. Wan, Z. Ma, B. Li, K. Zhang, S. Cao, S. Zhang and Z. Shi, J. Am. Chem. Soc., 2006, 128, 7416 CrossRef CAS PubMed; (d) B. V. S. Reddy, L. R. Reddy and E. J. Corey, Org. Lett., 2006, 8, 3391 CrossRef CAS PubMed; (e) T.-S. Mei, X. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 10806 CrossRef CAS PubMed.
- (a) R. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510 CrossRef CAS PubMed; (b) D.-H. Wang, T.-S. Mei and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 17676 CrossRef CAS PubMed; (c) D.-H. Wang, K. M. Engle, B.-F. Shi and J.-Q. Yu, Science, 2010, 327, 315 CrossRef CAS PubMed.
- (a) A. Lazareva and O. Daugulis, Org. Lett., 2006, 8, 5211 CrossRef CAS PubMed; (b) G. Cai, Y. Fu, Y. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 7666 CrossRef CAS PubMed; (c) B. Haffemayer, M. Gulias and M. J. Gaunt, Chem. Sci., 2011, 2, 312 RSC.
- (a) Y. Lu, D. Leow, X.-S. Wang, K. M. Engle and J.-Q. Yu, Chem. Sci., 2011, 2, 967 RSC; (b) X.-S. Wang, Y. Lu, H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 12203 CrossRef CAS PubMed.
- (a) K. Graczyk and L. Ackermann, Org. Lett., 2012, 14, 4110 CrossRef CAS PubMed; (b) S. H. Park, J. Y. Kim and S. Chang, Org. Lett., 2011, 13, 2372 CrossRef CAS PubMed; (c) K. Padala, P. Madasamy and M. Jeganmohan, Chem. Commun., 2012, 48, 7140 RSC.
- (a) X.-S. Zhang, Q.-L. Zhu, F.-X. Luo, G. Chen, X. Wang and Z.-J. Shi, Eur. J. Org. Chem., 2013, 6530 CrossRef CAS; (b) S. Rakshit, C. Grohmann, T. Besset and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2350 CrossRef CAS PubMed; (c) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064 CrossRef CAS PubMed; (d) F. Jafarpour, H. Hazrati, N. Mohasselyazdi, M. Khoobi and A. Shafiee, Chem. Commun., 2013, 49, 10935 RSC; (e) J. Karthikeyan, R. Haridharan and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 12343 CrossRef CAS PubMed; (f) L. Chan, X. Meng and S. Kim, J. Org. Chem., 2013, 78, 8826 CrossRef CAS PubMed; (g) G. Li, D. S. Leow, L. Wan and J.-Q. Yu, Angew. Chem., Int. Ed., 2012, 52, 1247 Search PubMed.
- S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451 CrossRef CAS PubMed.
- T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1148 CrossRef PubMed.
- L. V. Desai, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 9542 CrossRef CAS PubMed.
- (a) A. R. Dick, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 2300 CrossRef CAS PubMed; (b) D. Kalyani, N. R. Deprez, L. V. Desai and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 7330 CrossRef CAS PubMed.
- R. Giri, J. Liang, J.-G. Lei, J.-J. Li, D.-H. Wang, X. Chen, I. C. Naggar, C. Guo, B. M. Foxman and J.-Q. Yu, Angew. Chem., Int. Ed., 2005, 44, 7420 CrossRef CAS PubMed.
- (a) Q. Li, S.-Y. Zhang, G. He, W. A. Nack and G. Chen, Adv. Synth. Catal., 2014, 356, 1544 CrossRef CAS; (b) S.-Y. Zhang, G. He, Y.-S. Zhao, K. Wright, W. A. Nack and G. Chen, J. Am. Chem. Soc., 2012, 134, 7313 CrossRef CAS PubMed.
- R. K. Rit, M. R. Yadav and A. K. Sahoo, Org. Lett., 2012, 14, 3724 CrossRef CAS PubMed.
- G. Shan, X.-L. Yang, Y. Zong and Y. Rao, Angew. Chem., Int. Ed., 2013, 52, 13106 CrossRef.
- (a) Q. Wang, J. Han, C. Chao, J.-Y. Zhang, D.-Q. Shi and Y.-S. Zhao, Chem. Sci., 2014, 5, 4962 RSC; (b) J. Han, P. Liu, C. Wang and Y.-S. Zhao, Org. Lett., 2014, 16, 5682 CrossRef CAS PubMed; (c) C. Wang, C.-P. Chen, J.-Y. Zhang, Y.-M. Yao and Y.-S. Zhao, Angew. Chem., Int. Ed., 2014, 53, 9884 CrossRef CAS PubMed.
- J. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs and J.-Q. Yu, Science, 2014, 343, 1216 CrossRef CAS PubMed.
- (a) J. B. Gary and M. S. Sanford, Organometallics, 2011, 30, 6143 CrossRef CAS; (b) B. Lian, L. Zhang, G. A. Chass and D.-C. Fang, J. Org. Chem., 2013, 78, 8376 CrossRef CAS PubMed; (c) L. Guo, Y. Xu, X. Wang, W. Liu and D. Lu, Organometallics, 2013, 32, 3780 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00114e |
| This journal is © The Royal Society of Chemistry 2015 |