Two-photon pumped emission of polymeric thin film doped with dicyanopyranone derivative†
Zheng Gaoab and
Yi Chen*a
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, The Chinese Academy of Sciences, Beijing, 100190, China. E-mail: yichen@mail.ipc.ac.cn; Fax: +86 10 6487 9375; Tel: +86 10 8254 3595
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 16th February 2015
Abstract
Two-photon pumped up-conversion emission of polymeric thin film doped with an organic fluorophore dye (1) is described. It is found that 1 exhibits strong two-photon pumped up-conversion emission in polymeric thin film, and the mechanism shows that the up-conversion emission results from efficient two-photon absorption.
The design and control of the up or down frequency-converting emission constitutes a major area for fluorescent dyes.1 Up-conversion emission is an anti-Stokes-shifted process (down-conversion emission is a Stokes-shifted process) that consists of converting two or more photons of low energy into one photon of higher energy. Typically, two near-infrared photons are absorbed, and a visible photon is emitted.
Up-conversion emission has attracted a great deal of attention for its potential wide range of applications including display, laser, and security.2–5 Special opportunities are found in biotechnology.6–10 In this case, the higher penetration through the tissue of a near-infrared pumping source is an advantage compared with the UV source used for a down-conversion process.11 Recently, two-photon pumped up-converted lasing12,13 is of increasing interest since it is a new approach to accomplish frequency up-conversion of coherent light without phase-matching requirements which are difficult to be fulfilled in common approaches (second-harmonic generation or third-harmonic generation) of frequency up-conversion. The molecule systems which exhibit up-conversion emission in solid state are usually from lanthanide complexes,14–17 and a few examples are based on organic small molecules.18,19 This is because organic fluorophores exhibit strong fluorescence in dilute solutions but no or weak fluorescence in the solid state as a consequence of the tight molecular packing in the pure microcrystalline state or amorphous solid phase that usually leads to significant self-quenching.20
Organic small molecules with donor and acceptor π-conjugation system have attracted increasing interest as a promising class of nonlinear optical material through a two or more photon absorption process.21–25 As compared to inorganic materials, organic materials exhibit more efficient nonlinear optical properties and tenability within a quite broad spectral range. Moreover, organic materials offer easier processing, faster response time, and lower lasing threshold. The challenge is the development of the ideal solid organics with large two-photon absorption and efficient two-photon induced fluorescence since many chromophoric organics are highly emissive in their dilute solutions but only weakly luminescent when the concentration is high, especially in the solid state. Recently, the impressive aggregation-induced emission (AIE) or aggregation-induced emission enhancement (AIEE) have been discovered,26,27 and some elegant organic systems with AIE or AIEE have been developed.28–31 In this paper, we employ a dicyanopyranone derivative 1 which consists of bis-(D–π–A) structure (Scheme 1) as target molecule. It is found that 1 exhibits a strong up-conversion emission in polymeric thin film. As compared to the reported up-converting organic dyes, 1 has some merits include facile preparation, easy process, red emission, and good photo-stability.
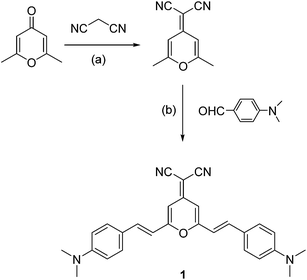 | ||
| Scheme 1 Chemical structure of 1 and its synthetic route. Reagents and conditions: (a) piperidine cat., dry ethanol, 60 °C, 90%; (b) piperidine cat., dry acetonitrile, 40 °C, 60%. | ||
Synthetic route for 1 is presented in Scheme 1, the detailed procedures see ESI.† 1 is composed with a typical donor–π–acceptor (D–π–A) structure, which exhibits intramolecular charge transfer characteristics.32,33 To confirm the intramolecular charge transfer of 1, the linear optical properties of 1 in dilute solutions (20 μM) are measured and their photophysical data are reported in Table 1. In dichloromethane (DCM) solution (Fig. 1), 1 showed multiple absorption bands at 332 nm, 368 nm, and 482 nm, respectively. Both 332 nm and 368 nm may be deduced to a localized aromatic π → π* transition, and 482 nm to intramolecular charge transfer transition. Consistent with the predicted trend, the maximal absorption of 1 exhibits solvatochromic effect with different solvents, as shown in table, the maximal absorption of 1 was red-shifted from 470 nm to 490 nm when the solvent was changed from toluene (small polarity) to DMSO (large polarity), which suggests a significant intramolecular charge transfer in solvent with large polarity.
| Solvent | λmax/nm | εmax/M−1 cm | λem/nm | ϕf | Δλ/nm |
|---|---|---|---|---|---|
| Toluene | 470 | 5.8 × 104 | 565 | 0.32 | 95 |
| DCM | 482 | 4.1 × 104 | 606 | 0.27 | 124 |
| Acetone | 480 | 7.0 × 104 | 609 | 0.02 | 129 |
| EtOH | 482 | 6.1 × 104 | 611 | 0.02 | 129 |
| DMSO | 490 | 6.2 × 104 | 607 | 0.003 | 117 |
Upon excitation the solution of 1 with 490 nm light, an orange fluorescence with the maximum emission wavelength at 606 nm was detected, by using rhodamine B (ϕf = 0.31 in H2O) as reference, a moderate fluorescence quantum yield (ϕf = 0.27) was obtained. Further investigation finds that 1 exhibits the characteristic of intramolecular charge transfer: the emission wavelength is red-shifted and the fluorescent intensity is decreased with increase of the polarity of solvents, as shown in Table 1, the emission wavelength of 1 was red-shifted from 565 nm to 606 nm when the solvent was changed from toluene to dichloromethane, but the fluorescence quantum yield was decreased from 0.32 (in toluene) to 0.27 (in dichloromethane). The positive solvatochromism results from the stabilization of the excited state by polar solvents due to strong interaction between molecules in the excited state and surrounding solvents.34 The decreased fluorescence may be attributed to the conversion of the excited state from the locally excited state to the intramolecular charge transfer state. As shown in table, a very weak emission of 1 was detected when 1 was dissolved in EtOH or DMSO. Further investigation found that the fluorescence of 1 was dramatically quenched (fluorescence intensity decreased 93%) as 10% water fractions in the THF–H2O mixture solution (see ESI†). The quenched emission of 1 in large polarity solvents is probably due to strong intramolecular charge transfer in excited state and resulted in charge separated, in which nonradiative transition is main way in relaxation process.35,36
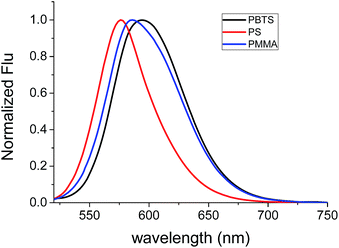
A strong fluorescence of 1 in polymeric thin film (thickness of thin film: 10 μM) was detected. As presented in Fig. 2, the maximum absorption of 1 in PMMA thin film appeared around 485 nm. Upon excitation with 490 nm, the maximum emission appeared at 580 nm, which occurred 26 nm blue-shift by comparison to that in DCM solution. The blue-shifted emission is as a result of the decrease of the polarity of polymeric media, which was confirmed by the emission of 1 in different polymeric thin film (Fig. 3), it was found that the emission of 1 was red-shifted from 574 nm to 594 nm when polymeric media was changed from polystyrene (PS, small polarity) to polybutylene terephthalate (PBT, large polarity). 1 showed strong emission in polymeric thin film and the large absolute fluorescence quantum yields (ϕf = 55%) was obtained by using an integrating sphere measure when 1 doped in PS thin film (Table 2). As shown in Table 2, 1 also exhibited the characteristic of intramolecular charge transfer in polymeric thin film: the emission wavelength was red-shifted from 574 nm to 594 nm when the polymeric media was changed from PS to PBT, and fluorescence quantum yield was decreased from 55% to 26%.
| Polymer | λmax/nm | λem/nm | ϕf | Δλ/nm |
|---|---|---|---|---|
| PS | 478 | 574 | 55 | 96 |
| PMMA | 484 | 580 | 28 | 96 |
| PBT | 485 | 594 | 26 | 109 |
Aggregation behaviour and photo-stability of 1 in PMMA thin film were explored. The preliminary investigation found that high concentration of 1 in polymeric thin film resulted in the quench of the fluorescence. As shown in Fig. 4, the emission wavelength was red-shifted from 586 nm to 592 nm when the concentration of 1 in PMMA thin film was increased from 0.25 mg mL−1 to 0.75 mg mL−1 whereas the fluorescence intensity was decreased almost a half. The red-shift of emission and the decrease of fluorescence intensity are probably as a consequence of the molecular packing (aggregation) in polymeric thin film. Photo-stability experiment showed that 1 in PMMA thin film exhibited good resistance to photobleaching, less than 5% degradation was detected by absorption and fluorescence spectra (see ESI†) when 1-PMMA thin film was irradiated for 60 min under UV light (λ = 365 nm, power: 3.7 mW cm−2).
The two-photon induced up-conversion emission of 1 was carried out in 1-PMMA thin film with 960 nm Ti-sapphire femtosecond pulse laser (50 mW). As showed in Fig. 2, 1-PMMA thin film exhibited significant absorption in the range of 300–600 nm with the maximum peak at 485 nm, but no or very weak absorption beyond 650 nm. Upon excitation with 960 nm light, a strong orange fluorescence with the maximum emission wavelength at 600 nm was detected (Fig. 5), as compared to the emission with one-photon excitation, the two-photon emission peak of 1-PMMA thin film is red-shifted by 20 nm due to reabsorption effect.37
To insight into the up-conversion emission mechanism of 1 in polymeric thin film, the two-photon absorption cross section of 1 is performed. Direct measurement of two-photon absorption cross section of compounds in solid state is so far technically challenging in the field of nonlinear optics, therefore, the solution of 1 in dichloromethane was measured (experimental details see ESI†) instead as an approximation of the property of 1 in PMMA thin film. Fig. 6 represented the two-photon absorption spectra of 1 in dichloromethane solution (9.2 × 10−4 mol L−1) with different excitation wavelength, as shown in Fig. 6 two main peaks are observed: the large one around 960 nm (760 GM, 1 GM = 10−50 cm4 photon−1 s−1) and the small one at 730 nm (380 GM), which corresponded to the peaks at 485 nm and 368 nm, respectively, in one-photon absorption spectral (Fig. 2). The results indicated that 1 exhibited two-photon absorption properties, which resulted in two-photon induced up-conversion emission of 1 in PMMA thin film.
 | ||
| Fig. 6 Two-photon absorption spectra in 700–1050 nm region of 1 (9.2 × 10−4 M) in DCM solution (Ti-sapphire laser, 120 fs, 82 MHz). | ||
Conclusions
In summary, two-photon pumped up-conversion emission based on a small organic molecule in polymeric thin film has been developed. It has demonstrated that dicyanopyranone derivative with bis (D–π–A) structure shows strong emission in polymeric thin film. It has also demonstrated that the dicyanopyranone derivative exhibits two-photon absorption property, with red and near-infrared light excitation, a strong up-conversion emission in thin film is observed.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no 91123032 and 21302197).Notes and references
- G. Chen, C. Yang and P. N. Prasad, Acc. Chem. Res., 2013, 46, 1474 CrossRef CAS PubMed.
- F. Heine, E. Heumann, T. Danger, T. Schweizer, G. Huber and B. Chai, Appl. Phys. Lett., 1994, 65, 383 CrossRef CAS PubMed.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CAS.
- L. W. Yang, H. L. Han, Y. Y. Zhang and J. X. Zhong, J. Phys. Chem. C, 2009, 113, 18995 CAS.
- B. Neupane, L. Zhao and G. Wang, Nano Lett., 2013, 13, 4087 CrossRef CAS PubMed.
- G. S. He, L. S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245 CrossRef CAS PubMed.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161 CrossRef CAS PubMed.
- M. Yu, F. Li, Z. Chen, H. Hu, C. Zhan, H. Yang and C. Huang, Anal. Chem., 2009, 81, 930 CrossRef CAS PubMed.
- L. Li, P. Wu, K. Hwang and Y. Lu, J. Am. Chem. Soc., 2013, 135, 2411 CrossRef CAS PubMed.
- N. Niu, F. He, P. Ma, S. Gai, G. Yang, F. Qu, Y. Wang, J. Xu and P. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 3250 CAS.
- G. S. Yi, H. C. Lu, S. Y. Zhao, G. Yue, W. J. Yang, D. P. Chen and L. H. Guo, Nano Lett., 2004, 4, 2191 CrossRef CAS.
- Q. D. Chen, H. H. Fang, B. Xu, J. Yang, H. Xia, F. P. Chen, W. J. Tian and H. B. Sun, Appl. Phys. Lett., 2009, 94, 201113 CrossRef PubMed.
- G. S. He, T. C. Lin, V. K. S. Hsiao, A. N. Cartwright, P. N. Prasad, L. V. Natarajan, V. P. Tondiglia, R. Jakubiak, R. A. Vaia and T. J. Bunning, Appl. Phys. Lett., 2003, 83, 2733 CrossRef CAS PubMed.
- Y. Li, J. Zhang, X. Zhang, Y. Luo, X. Ren, H. Zhao, X. Wang, L. Sun and C. Yan, J. Phys. Chem. C, 2009, 113, 4413 CAS.
- G. Wang, Q. Peng and Y. Li, J. Am. Chem. Soc., 2009, 131, 14200 CrossRef CAS PubMed.
- H. Zhu, X. Chen, L. M. Jin, Q. J. Wang, F. Wang and S. F. Yu, ACS Nano, 2013, 7, 11420 CrossRef CAS PubMed.
- P. Zuo, X. Hong, Y. Ding, Z. Zhang, X. Chu, T. Shaymurat, C. Shao and Y. Liu, J. Phys. Chem. C, 2012, 116, 5787 Search PubMed.
- H. Fang, Q. Chen, J. Yang, H. Xia, B. Gao, J. Feng, Y. Ma and H. Sun, J. Phys. Chem. C, 2010, 114, 11958 CAS.
- R. R. Islangulov, J. Lott, C. Weder and F. N. Castellano, J. Am. Chem. Soc., 2007, 129, 12652 CrossRef CAS PubMed.
- M. M. Sartin, A. J. Boydston, B. L. Pagenkopf and A. J. Bard, J. Am. Chem. Soc., 2006, 128, 10163 CrossRef CAS PubMed.
- A. R. Guzman, M. R. Harpham, O. Süzer, M. M. Haley and T. G. Goodson III, J. Am. Chem. Soc., 2010, 132, 7840 CrossRef CAS PubMed.
- R. L. Gieseking, S. Mukhopadhyay, C. Risko and J. Brédas, ACS Photonics, 2014, 1, 261 CrossRef CAS.
- Y. V. Pereverzev, K. N. Gunnerson, O. V. Prezhdo, P. A. Sullivan, Y. Liao, B. C. Olbricht, A. J. P. Akelaitis, A. K. Jen and L. R. Dalton, J. Phys. Chem. C, 2008, 112, 4355 CAS.
- F. Castet, V. Rodriguez, J. Pozzo, L. Ducasse, A. Plaquet and B. Champagne, Acc. Chem. Res., 2013, 46, 2656 CrossRef CAS PubMed.
- R. R. Tykwinski, U. Gubler, R. E. Martin, F. Diederich, C. Bosshard and P. Günter, J. Phys. Chem. B, 1998, 102, 4451 CrossRef CAS.
- B. Z. Tang, X. W. Zhan, G. Yu, P. P. S. Lee, Y. Q. Liu and D. B. Zhu, J. Mater. Chem., 2001, 11, 2974 RSC.
- Y. N. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC.
- V. Vij, V. Bhalla and M. Kumar, ACS Appl. Mater. Interfaces, 2013, 5, 5373 CAS.
- X. Zhang, X. Zhang, B. Yang, Y. Zhang and Y. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 3600 CAS.
- M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858 RSC.
- C. Shi, Z. Guo, Y. Yan, S. Zhu, Y. Xie, Y. S. Zhao, W. Zhu and H. Tian, ACS Appl. Mater. Interfaces, 2013, 5, 192 CAS.
- Z. Q. Guo, W. H. Zhu and H. Tian, Chem. Commun., 2012, 48, 6073 RSC.
- Y. Zhang, P. Gu, J. Zhou, Y. Xu, W. Liu, Q. Gu, D. Chen, N. Li, Q. Xu and J. Lu, J. Mater. Chem. C, 2014, 2, 2082 RSC.
- X. Sun, Y. Liu, X. Xu, C. Yang, G. Yu, Z. Zhao, W. Qin, Y. Li and D. Zhu, J. Phys. Chem. B, 2005, 109, 10786 CrossRef CAS PubMed.
- R. R. Hu, E. Lager, A. Aguilar-Aguilar, J. Z. Liu, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, Y. C. Zhong, K. S. Wong, E. Pena-Cabrera and B. Z. Tang, J. Phys. Chem. C, 2009, 113, 15845 CAS.
- M. Mao, M. G. Ren and Q. H. Song, Chem.–Eur. J., 2012, 18, 15512 CrossRef CAS PubMed.
- T. Lin, G. He, P. N. Parasad and L. Tan, J. Mater. Chem., 2004, 14, 982 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characteristic data of 1, experimental for two-photo excitation and two-photon absorption cross section. See DOI: 10.1039/c5ra00095e |
| This journal is © The Royal Society of Chemistry 2015 |