Effective photoreduction of a nitroaromatic environmental endocrine disruptor by AgNPs functionalized on nanocrystalline TiO2†
Abstract
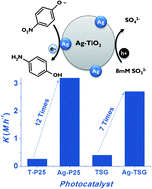
Unprecedented photoactivity of silver nanoparticles photodeposited on nanocrystalline TiO2 for the efficient reduction of 4-nitrophenol at room temperature is reported. The use of Na2SO3 as a harmless scavenger agent for the reduction of a nitroaromatic endocrine disruptor yields a valuable 4-aminophenol reagent.

 Please wait while we load your content...
Please wait while we load your content...