Microwave synthesis of a CoSe2/graphene–TiO2 heterostructure for improved hydrogen evolution from aqueous solutions in the presence of sacrificial agents
Kefayat Ullah,
Zhu Lei,
Shu Ye,
Asghar Ali and
Won-Chun Oh*
Department of Advanced Materials Science & Engineering, Hanseo University, Seosan-si, 356-706, Chungnam-do, Korea. E-mail: wc_oh@hanseo.ac.kr; Fax: +82-41-688-3352; Tel: +82-41-660-1337
First published on 4th February 2015
Abstract
A heterogeneous material consisting of a tube type TiO2 was grown in the presence of a CoSe2/graphene hybrid, as a high-performance photocatalyst material, through a fast microwave-assisted technique. The prepared composites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Raman spectroscopic analysis, UV-vis absorbance spectra and UV-vis diffuse reflectance spectra (DRS) analysis. The hydrogen evolution rate for ternary composites was found to be markedly high compared to bare TiO2 and binary CoSe2/graphene composites. This extraordinary photocatalytic activity for hydrogen evolution arises from the positive synergistic effect between the CoSe2 and graphene components in our heterogeneous system. The optical properties were also found to be affected by the different weight% of graphene in the composites by observing their respective band gaps from the DRS spectra.
1. Introduction
Hydrogen production from water splitting through a graphene based semiconductor photocatalyst has attracted amassing attention in the past due to its great potential in obtaining renewable energy.1 Heterogeneous catalyst systems consist of two semiconductor materials and a mediator/support material or the class of photocatalyst for solar hydrogen production. The significant advantage of heterogeneous systems is the separation of photoexcited electrons and holes with strong redox potentials on different semiconductors. So far in these systems, a new reversible donor–acceptor pair has been introduced as a redox mediator in the reaction solution, to facilitate the charge transfer between the two semiconductor materials. These mediators are mainly Fe3, Fe2, IO3 etc.2–5 Similarly a solid state electron mediator has also been introduced to reduce the backward reduction involving the redox mediator.6,7 The introduction of these metallic components as electron mediators has several drawbacks including high price, metallic pollution, corrosion etc. Therefore a suitable electron mediator is needed to design a new heterogeneous system for an improved catalytic effect towards hydrogen production.Recently graphene has been used very extensively in combination with various semiconductor materials. The surface functionalities, large surface area and stability of graphene make it a suitable candidate to ensure the attachment of semiconductor materials on its surface and facilitate the charge transfer mechanism. The oxygen functionalities affect the particle size and shape of the guest material on the graphene sheet. The guest material’s properties and shape are very important in the mechanism of photocatalyst systems. The surface modifications of graphene through these semiconductor materials open up new ways to establish and design semiconductor photocatalysts with desirable properties.
Several scientists have already reported that graphene can act as a photosensitizer to extend the light response or it can reduce the band gap of wide semiconductors, such as TiO2 and ZnO, towards the visible range of electromagnetic spectrum.8,9 Recently, special attention has been paid to coupled graphene/TiO2 systems due to the high surface area and excellent charge conductivity of graphene and the non-toxicity, low price and environmentally friendly behavior of TiO2.10–12 It was observed that graphene can effectively transfer and store excited electrons when coupled with a TiO2 semiconductor. Due to π–π interactions it can also enhance the affinity with organic pollutants and can help to enhance the catalytic process. The fabrication of new photocatalysts needs special attention to meet the environmental requirements. i.e. using stable, non-toxic, simple and fast synthesis techniques. A heterogeneous photocatalyst system can produce better catalytic activity due to the synergistic effect between the two components. The energy level of the system can be adjusted to the desired level by quantum confinements. The transfer of electrons in the heterogeneous system between two catalysts is the main feature for the production of H2 or other mineral products. The main challenge in these systems is the introduction of an electron mediator with stable conducting properties and having great interfacial contact.13,14
In spite of these promising properties, the main problem in ternary systems is the agglomeration of nanoparticles on the graphene sheet due to a large number of functional groups on the graphene surface. These agglomerations lead towards a lower surface area and poor interfacial contact. Therefore homogenous distribution of nanoparticles, high surface area and better interfacial contact are the requirements of ternary photocatalyst materials.15
In this work we report a fast and facile route for the preparation of a CoSe2/graphene supported TiO2 photocatalyst through a microwave-assisted technique. In this process, cobalt acetate and selenium salt were mixed together in 100 mL ethylene glycol, followed by graphene oxide. The resulting solution was irradiated by microwaves for 300 s, followed by mixing the TiO2 precursor material under appropriate conditions. During microwave irradiation, the partial reduction of graphene oxide into graphene and attachment of CoSe2 nanoparticles and TiO2 nanotubes on the graphene sheets was observed in ethylene glycol. The photocatalytic activities of the as-prepared nanocomposites were tested for the production of hydrogen from aqueous solutions containing Na2SO3/Na2S and methanol as sacrificial reagents.
2. Experimental section
2.1. Materials
Titanium(IV) n-butoxide (TNB, C16H36O4Ti), used as a titanium precursor, sodium sulfide pentahydrate (Na2S·5H2O) and sodium sulfite (Na2SO3) were purchased from Samchun Pure Chemical Co., Ltd., Korea. Selenium powder (Se, 99%) and ammonium hydroxide (NH4OH, 25–28) were purchased from Daejung Chemicals Co., Ltd., Korea. Cobalt chloride (CoCl2) and ethylene glycol were purchased from Dae-Jung Chemicals & Metal Co., Ltd., Korea. All chemicals were used without further purification and all experiments were carried out using distilled water.2.2. Synthesis of cobalt selenide
Cobalt selenide was synthesized through a fast microwave-assisted technique. In a typical synthesis, 1.5 g of anhydrous sodium sulfite (Na2SO3) and 0.3 g of crude selenium (Se) powder were mixed together in 300 mL of ethylene glycol under vigorous magnetic stirring. The solution was stirred vigorously for 1 h at 50 °C to ensure homogeneous mixture to achieve the selenium salt. In the next step, 0.5 mmol of cobalt acetate was added to the above solution and stirred for 20 minutes to attain a stable solution. The obtained solution was finally transferred to a 500 mL reaction vessel and placed in a conventional microwave oven. The solution was then irradiated with microwaves for 5 s on and 5 s off for 300 s. The mixture was then cooled to room temperature, filtered with Whatman filter paper and heat treated for 5 h at 90 °C to obtain a dark brown CoSe2 powder.2.3. Synthesis of CoSe2/graphene nanocomposites
The GO was prepared through a modified Hoffman method reported elsewhere.16 GO (200 mg) was dispersed in a ethylene glycol (EG) solution (100 mL) under vigorous stirring to form solution A. Cobalt selenide was prepared as explained above. 0.9 molar solution of cobalt selenide was prepared to form solution B. Solutions A and B were mixed together under stirring for several minutes and transferred into a 500 mL reaction vessel placed in a conventional microwave oven. The solution was then irradiated with microwaves for 5 s on and 5 s off for 300 s, and cooled to room temperature before being filtered with Whatman filter paper. The resultant powder was washed 3 times with distilled water and transferred into a dry oven for 6 hours at 90 °C. The powder was then heat treated at 500 °C for 1 h in an electric furnace. The sample obtained was labeled as CoSe2/G nanocomposites.2.4. Synthesis of CoSe2/graphene–TiO2 nanocomposites
CoSe2/graphene–TiO2 nanocomposites were obtained by following the above method. A borosilicate glass sealed reaction vessel specially designed for microwave techniques, with a diameter of 8 cm and a height of 10 cm, was used. 200 mg graphene oxide and a desired amount of TNB, as a titanium precursor, were dispersed in 300 mL ethylene glycol for 30 minutes to attain a homogenous mixture, called solution A. In the next step, 1 g of anhydrous sodium sulfite (Na2SO3) and 0.2 g of crude selenium powder (Se) were vigorously stirred in 200 mL of ethylene glycol for 30 min to attain a homogenous solution. This was followed by the addition of a desired amount of cobalt acetate with vigorous stirring for 1 h at 35 °C to ensure homogenous mixing to form a stable suspension B. A and B were mixed together under stirring for several minutes, transferred into a 500 mL reaction vessel and placed in a conventional microwave oven. The solution was then irradiated with microwaves for 5 s on and 5 s off for 300 s, and cooled to room temperature before being filtered with Whatman filter paper. The resultant powder was washed 3 times with distilled water and transferred into a dry oven for 6 h at 90 °C. The powder was then heat treated at 500 °C for 1 h in an electric furnace. The weight ratios of GO to CoSe2 and TiO2 were taken as 0.5%, 1.5% and 2.5% and the obtained samples were labeled as CGT1, CGT2 and CGT3 respectively.2.5. Photocatalytic hydrogen evolution system
The photocatalytic reaction was carried out at room temperature. The photocatalyst powder, 50 mg CoSe2/graphene–TiO2, was dispersed by a magnetic stirrer in 200 mL aqueous solutions containing 0.06 mol L−1 Na2S, 0.04 mol L−1 Na2SO3 and 20% methanol as sacrificial reagents. A 356 nm UV light source was employed at a distance of 20 cm from the glass reactor. The amount of hydrogen gas evolved was detected by a Minimax (X13010683) XP H2 sensor.2.6. Characterization
To determine the crystal phase and the composition of the as-prepared CoSe2/graphene–TiO2 samples, XRD characterization was carried out at room temperature using XRD (Shimata XD-D1, Japan) with Cu Kα radiation (λ = 1.54056 Å) in the range of 2θ = 10–80° at a scan speed of 1.2° m−1. Transmission electron microscopy (TEM, JEOL, JEM-2010, Japan) was used to observe the surface state and structure of the photocatalyst composites at an acceleration voltage of 200 kV. TEM was also used to examine the size and distribution of the CoSe2 particles deposited on the graphene sheet. Diffuse reflectance spectra were obtained using a scanning UV-vis spectrophotometer (Neosys-2000) equipped with an integrating sphere assembly. Scanning electron microscopy (SEM, JSM-5200 JOEL, Japan) was used to observe the surface state and morphology of the prepared nanocomposites. The morphology of the samples was studied using energy dispersive X-ray spectroscopy (EDX), which was also employed for elemental analysis. X-ray photoelectron spectroscopy (XPS) was performed using a VG scientific VISACA lab 2000 instrument and a monochromatic Mg X-ray radiation source. Raman spectra of the samples were observed using a spectrometer (Jasco Model Name NRS-3100) with an excitation laser wavelength of 532.06 nm.3. Results and discussion
3.1. Growth and characterization of CoSe2/graphene–TiO2 nanocomposites
The synthesis method for our nanocomposites is shown in Scheme 1. As it is already known that microwave radiation need a polar solvent for synthesis of nanomaterials. We consider ethylene glycol as a solvent in our experiments. These polar solvents absorb microwave energy and limit localized heating and causes decomposition The advantage of microwave synthesis are, i.e., short reaction time, saving of long usage of power, extra cooled water for reflux and closed vessel to release minimum toxic gases to environment.17–19 The high energy microwave generates hot spot, with extremely high localized temperature and pressure which tends to accelerate the nanoparticles and simultaneous attachment occur on graphene sheet.20,21The crystal phases were analyzed through XRD techniques, as depicted in Fig. 1. The bare TiO2 and CoSe2/G have a diffraction pattern that corresponds to the cubic cobalt selenide structure, which confirms the formation of a cubic crystalline structure on the graphene sheet.22 Fig. 1 depicts the XRD pattern of the CoSe2/G nanocomposites. The diffraction peak of CoSe2 (111) and graphene (002) are located at 26° at 2θ. It is difficult to distinguish both peaks as a result of the broad reflection from the CoSe2. The CoSe2/G composites with different compositions exhibit the characteristic (111), (200), (210), (211), (220), and (311), (230), (321), (400), (421), (511) reflections that correspond to the crystal phase (JCPDS PDF#: 00-065-3327).
 | ||
| Fig. 1 (a–e), XRD pattern of CoSe2/graphene–TiO2 nanocomposites; (a) TiO2, (b) CoSe2/G, (c) CGT1, (d) CGT2 and (e) CGT3. | ||
The TiO2 diffraction peak (101) and graphene (002) peaks are located at the same 2θ values. The nanocomposites contain characteristic reflections (101), (004), (200), (105), (211), and (220) that correspond to the anatase crystal phase (JCPDS PDF#: 00-021-1272). Fig. 1 shows a slight decrease in the intensity of the CGT1 nanocomposites as compare to TiO2 and CoSe2/G. The distortion is not completely related with the decrease in intensity. However, in light of the reported results, we assume that the suppression of the peaks leads towards distortion of the crystalline phase of the semiconductor materials on the graphene sheet.23,24 The interaction of nanoparticles with the graphene surface creates a phonon confinement effect by decreasing the probability of spherical shaped nanoparticles. Therefore we observed some tube type TiO2 nanoparticles in the composite, as depicted in the TEM images of the ternary composites.
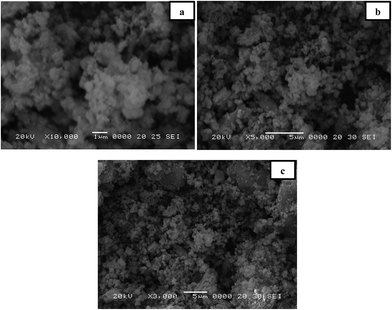
We examined the morphological structure of our nanocomposites by scanning electron microscopy, as shown in Fig. 2. The CoSe2/graphene nanocomposites depict the plate like morphology of graphene, while the particle size and shape are difficult to visualize in SEM. The SEM image was taken to describe the overall morphology and shape of graphene in the composite. From this figure the overall structure can be clearly predicted with graphene as a sheet-like structure broken off in different directions. Both images in Fig. 2(a and b), show that CoSe2 may be a spherical shape with partial agglomeration. After microwave treatment, the sheet morphology is retained and the graphene surfaces are covered with CoSe2 nanoparticles. The anchoring of nanoparticles is very helpful to overcome the interactions between the functionalities on the graphene surface. Fig. 3(a–c) display the CoSe2/graphene–TiO2 nanocomposites. From these images we can clearly observe the difference between binary and ternary composites. After attachment of TiO2 the brighter spot arises in the ternary composite. We assumed that TiO2 also resides on the graphene surface supporting CoSe2 nanoparticles. Further TEM images confirm the shape of these TiO2 and CoSe2 nanoparticles.
TEM analysis was carried out to further study the microscopic structural information of the binary CoSe2/graphene composites, as shown in Fig. 4(a and b). The images in Fig. 4(a and b) were taken with the same magnification so that the large surface structure and the overall history of the nanoparticles and graphene sheet were exposed. This image shows that the graphene sheets are well distributed, providing a large microsheet type plate structure for the CoSe2 nanoparticles. The overall image indicates that significantly less agglomeration of the nanoparticles on the graphene sheet is observed. Fig. 4(b) further confirms that almost spherical nanoparticles with uniform sizes can be observed on the graphene sheet.
Fig. 5 shows TEM images of the CGT2 nanocomposites. TEM analysis was carried out to further study the microscopic structural information of the ternary CoSe2/graphene–TiO2 nanocomposites, as shown in Fig. 5(a–d). The images in Fig. 5(a and b) demonstrate the 100 and 50 nm magnification images of the CGT2 nanocomposites. From these images tube type TiO2 can be seen with partial agglomeration. We assumed that the seeming agglomeration is due to the large size of the TiO2 nanotubes, with lengths of more than 100 nm. To further investigate the shape and size of the nanocomposites, we obtained high resolution TEM images with 20 and 5 nm magnifications, as shown in Fig. 5(c and d). As it can be seen from the high magnification images that the TiO2 nanotubes were found supporting CoSe2 nanoparticles on the graphene sheet. Thus, we can say that microwave-assisted synthesis of CoSe2/graphene–TiO2 is advantageous over other synthesis methods, and would be beneficial for the improved photocatalytic properties of these materials.
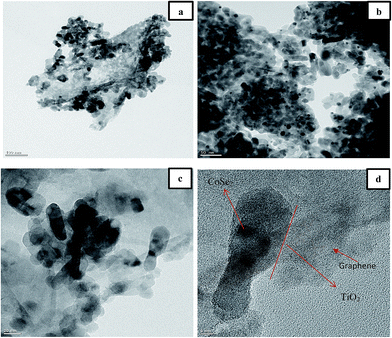 | ||
| Fig. 5 (a–d). TEM images of CGT2 nanocomposites: (a) 100 nm, (b) 50 nm, (c) 20 nm and (d) 5 nm maginification. | ||
The Raman spectra of the CoSe2/graphene–TiO2 nanocomposites show two prominent peaks corresponding to a D band and G band, as depicted in Fig. 6. The D band is induced by defects, while the G band is induced by sp2 carbon bonds. Fig. 6 depicts the micro Raman spectra of our nanocomposites with a G band and D band, corresponding to the vibration of a carbon atom in disorder or defects site and in plane vibration of sp2 bonded carbon atoms respectively.16 The difference in the Raman band intensity or shift provides information about the nature of the defects and C–C bonds.25,26 The peaks observed below 600 cm−1 in Fig. 6(a) are attributed to the CoSe2 crystal. The peaks around 200 cm−1 to 300 cm−1 wave number are attribute to an SeO trigonal phase, and the peaks around 410 cm−1 and 510 cm−1 seem to be associated with the cubic CoSe2 phase. The CoSe2/graphene–TiO2 nanocomposites in Fig. 6(b–d) show a slightly different Raman spectra compared to CoSe2/G.
The order of defects in graphene or graphene oxide can be reflected by the intensity ratio of the D to the G band. The calculated ID/IG of the CoSe2/graphene nanocomposites were found to be ∼0.981, while the value for the CGTs ternary nanocomposites was found to be 1.006. These empirical results depict that the introduction of TiO2 still affects the ratio and an increased amount of defects were found. Another important observation is the shift of G peaks towards a higher frequency compared to the binary CoSe2/graphene nanocomposites. The observed shift was found to be ∼20 cm−1. There are several possible origins for the shift of the Raman peaks. These may be due to the inclusion of semiconductors i.e. CoSe2 and TiO2 as dopants, laser excitation during the Raman experiment, and the electron–holes created during this excitation by the attached semiconductor materials.27–29 There are some characteristic peaks observed, located at 148, 390, 516, and 640 cm−1 corresponding to the Eg(1), B1g(1), A1g + B1g(2), and Eg(2) modes of anatase TiO2.30,31
A comparison of the absorption spectra of the CoSe2/graphene–TiO2 nanocomposites is displayed in Fig. 7(a–e). An enhanced absorbance towards the visible region >500 nm was observed with the ternary compounds. Meanwhile the graphene based CoSe2 gives an absorbance smaller than the ternary composites. This enhanced absorbance may be attributed to the optimum loading of the support material on graphene and the homogeneous distribution of nanoparticles by providing a large number of reaction sites. From the figure we can see that bare TiO2 has a sharp edge in the UV region, which has been improved by introducing it in the ternary graphene based materials. The unpaired π electron in graphene may cause the interaction with metal or semiconductors nanoparticles. Such an interaction might shift the band edge and will increase the light absorption towards the visible region.32 Lee et al. also observed such an effect with titanium nanoparticles and observed the optical response towards the visible region of the electromagnetic spectrum.33
The estimation of the band gap was carried out using the Kubelka–Munk transformation by converting the reflectance plot according to the Tauc condition. The following equation was proposed by Tauc, Davis, and Mott.
| (hνα)1/n = A(hν − Eg) | (1) |
| (hνF(R∞))2 = A(hν − Eg) | (2) |
 | ||
| Fig. 8 Transform Kubelka–Munk function verses photon energy: (a) CoSe2/G, (b) CGT1, (c) CGT3 and (d) CGT2. Inset: (e) TiO2. | ||
The band gap energies were found to be in the order of TiO2 (inset Fig. 8) > CoSe2/G > CGT1 > CGT3 > CGT2 of the CoSe2/graphene–TiO2 nanocomposites. The differences in the band gap energies of our nanocomposites may possibly be attributed to the distribution of CoSe2 particles and TiO2 nanotubes on the graphene sheet. Similarly the partial agglomeration can also affect the absorption properties and hence may result in the band gap variation. Another reason for the variation of the band gap energies may be the different amounts of graphene in the composites, which considerably affects the optical property of our ternary nanocomposites. Therefore, reduction in the band gap is observed for the nanocomposites.34,35 Further enhanced visible light absorption occurred on the ternary CoSe2/graphene–TiO2 photocatalyst, confirming effectively enhanced light-harvesting activity due to the synergistic effect of CoSe2 and graphene as co-modifiers.
3.2. Photocatalytic hydrogen production studies
The role of ternary graphene based materials in improving catalytic activity was studied by monitoring the photocatalytic hydrogen production from aqueous solutions containing Na2S/Na2SO3 and methanol as sacrificial reagents. The CGT2 composites of the ternary CoSe2/graphene–TiO2 composite gives an hydrogen evolution rate of 205 μmol h−1, which is greater than all the other samples in the composites, as depicted in Fig. 9. Furthermore the P25 and CoSe2/graphene nanosheets give a smaller value of hydrogen evolution rate than the ternary system. The value of hydrogen evolution rate for this bare (P25) and the binary system is almost 6 times and 1.5 times less than the ternary system, respectively. The comparison clearly shows that the graphene based ternary system greatly improves the catalytic properties, indicating a strong interaction between graphene and the attached semiconductor materials. There is no doubt that the spherical CoSe2 supported by the tube type TiO2, allows good interfacial contact with the graphene sheet by boosting the synergistic effect between CoSe2 and graphene sheet. Similarly the dependence of different amounts of graphene also plays a crucial role in catalytic hydrogen evolution. The optimal content of graphene in the CoSe2/graphene–TiO2 system is determined to be 1.5 wt%. The higher content may lead towards a smaller rate, probably due to the increased light absorption of graphene itself, which disparately harms the excitation of CoSe2 and TiO2 in the composites. | ||
| Fig. 9 Reaction time profile of H2 evolution under UV light illumination from an aqueous solution containing Na2S/Na2SO3 with CGT (1–3) composites and CoSe2/G and P25 as the photocatalysts. | ||
As compared to our previous report, the photocatalytic H2 evolution for Pt/graphene nanocomposites and P25 were found to be marginally smaller than the present nanocomposites with TiO2.36 Graphene, as an electron acceptor/transporter, can promote the separation of the photo-generated electron–hole pairs in the ternary system, efficiently transport the photo-generated electrons to the TiO2 valance band by creating a donor level to transfer to the conduction band and finally, improve the photocatalytic H2 production. The role of the sacrificial reagents is to provide electrons to consume the photo-generated holes to further increase the catalytic properties, which will help to increase the recombination time in our nanocomposites.37
The hydrogen evolution plot for the 20% methanol solution is shown in Fig. 10. A relatively low hydrogen evolution rate (190 μmol) was reported compared to the Na2S/Na2SO3 aqueous solution. The work function of graphene is higher than that of the reagent at the interface between CoSe2 and graphene. This means that the electron transfer from graphene was energetically favorable. Due to the high electron affinity of graphene resulting from the giant π–π conjugated structure, the electron transfer from CoSe2 to graphene continues until the two systems attain a charge equilibrium.38,39
 | ||
| Fig. 10 Hydrogen production rate from an aqueous solution containing 25% methanol with CGT (1–3) composites, CoSe2/G and P25 as the photocatalyst. | ||
The shaped double charge layers stabilize the accepted electrons and gather in the graphene network. This decelerates the continuous addition of electrons from the reagents to the graphene sheet. If the trapped electrons are not transferred well, they are forced to be transferred back to recombine with the electron donors, which would lower the catalytic efficiency. In the case of the methanol solution, the lower catalytic hydrogen may be due to the recombination of donor electrons in the catalytic process.40,41 In the case of the methanol solution, the CGT2 composite demonstrates an improved photocatalytic hydrogen evolution rate compared to the other nanocomposites.
Addressing the stability of the photocatalysts containing sulfides as a sacrificial reagent is very important because of the well-known process of photocorrosion of sulfides. A time circle experiment was carried out to estimate the stability of our ternary CoSe2/graphene–TiO2 system. The respective hydrogen evolution rate was found for the CGT2 nanocomposite in the range of 198 to 195.5 μmol h−1, after 5 cyclic tests as shown in Fig. 11. There is no obvious change in the activity of the composites after 5 cycles. This suggests that CoSe2/graphene–TiO2 has excellent stability and can be used for a continuous photocatalytic hydrogen evolution system. This great improvement and stability of our ternary graphene system can be understood as follows: an effective charge carrier system at the interface of graphene and CoSe2, which is provided by graphene itself, the synergistic effect between CoSe2 and graphene, and the excellent charge transfer ability of graphene can accept electrons form conduction band of CoSe2 and transfer it to TiO2 via graphene sheet. These electrons through the conduction band of TiO2 reduce water to H2, while the holes in CoSe2 oxidize water to O2, achieving a complete water splitting cycle through our designed photocatalyst material, as shown in Fig. 12. Similarly the sheet morphology of graphene can reduce the diffusion length of photoexcited electron and holes form the bulk to the surface for resultant oxidation and reduction reaction.
4. Conclusion
In summary the heterogeneous CoSe2/graphene–TiO2 were synthesized through a fast microwave-assisted technique. TEM images clearly indicate that tube type TiO2 is distributed on the surface of the graphene sheets supported by CoSe2 nanoparticles. As a result of the heterostructure, the promotion of the excited charge carriers at the interface of CoSe2 and graphene was found to be highest due to the synergistic effect of graphene and CoSe2 by increasing the recombination time. The graphene in the system acts as a solid state electron mediator to support the ternary system and will help to provide stable photocatalyst heterogeneous materials. The optical properties were also shown to be affected by the different weight% of graphene in the composites by observing their respective band gaps from the DRS spectra. The present study has cemented a new way of using graphene based materials for developing an efficient heterosystem for hydrogen production.Acknowledgements
This work was supported by the research foundation of Hanseo University in the year 2014. The authors are grateful to the University for financial support.References
- K. Maeda, M. Higashi, D. L. Lu, R. Abe and K. Domen, Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst, J. Am. Chem. Soc., 2010, 132, 5858–5868 CrossRef CAS PubMed.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3-/I-shuttle redox mediator under visible light irradiation, Chem. Commun., 2001, 2416–2417 RSC.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis, J. Photochem. Photobiol., A, 2002, 148, 71–77 CrossRef CAS.
- S. Hara and H. Irie, Band structure controls of SrTiO3 towards two-step overall water splitting, Appl. Catal., B, 2012, 115, 330–335 CrossRef PubMed.
- S. Hara, M. Yoshimizu, S. Tanigawa, L. Ni, B. Ohtani and H. Irie, Hydrogen and oxygen evolution photocatalysts synthesized from strontium titanate by controlled doping and their performance in two-step overall water splitting under visible light, J. Phys. Chem. C, 2012, 116, 17458–17463 CAS.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, All-solid-state Z-scheme in CdS–Au–TiO2 three-component nano junction system, Nat. Mater., 2006, 5, 782–786 CrossRef CAS PubMed.
- H. J. Yun, H. Lee, N. D. Kim, D. M. Lee, S. Yu and J. Yi, A combination of two visible-light responsive photocatalysts for achieving the Z-scheme in the solid State, ACS Nano, 2011, 5, 4084–4090 CrossRef CAS PubMed.
- C. Chen, W. Cai, M. Long, B. Zhou, Y. Wu, D. Wu and Y. Feng, Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction, ACS Nano, 2010, 4, 6425–6432 CrossRef CAS PubMed.
- J. S. Lee, K. H. You and C. B. Park, Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene, Adv. Mater., 2012, 24, 1084–1088 CrossRef CAS PubMed.
- P. Chen, T. Y. Xiao, Y. H. Qian, S. S. Li and S. H. Yu, A nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity, Adv. Mater., 2013, 25(23), 3192–3196 CrossRef CAS PubMed.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, P25–graphene composite as a high performance photocatalyst., ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Graphene-based semiconductor photocatalysts, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- N. Zhang, Y. Zhang, X. Pan, M. Q. Yang and Y.-J. Xu, Constructing ternary CdS–graphene–TiO2 hybrids on the flatland of graphene oxide with enhanced visible-light photoactivity for selective transformation, J. Phys. Chem. C, 2012, 116, 18023–18031 CAS.
- K. Ullah, Z. D. Meng, S. Ye, L. Zhu and W. C. Oh, Synthesis and characterization of novel PbS–graphene/TiO2 composite with enhanced photocatalytic activity, J. Ind. Eng. Chem., 2014, 20(3), 1035–1042 CrossRef CAS PubMed.
- N. Zhang, S. Liu and Y.-J. Xu, Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst, Nanoscale, 2012, 4, 2227–2238 RSC.
- K. Ullah, S. Ye, S.-B. Jo, L. Zhu, K.-Y. Cho and W.-C. Oh, Optical and photocatalytic properties of novel heterogeneous PtSe2–graphene/TiO2 nanocomposites synthesized via ultrasonic assisted techniques, Ultrason. Sonochem., 2014, 21, 1849–1857 CrossRef CAS PubMed.
- E.-P. Ng, D. T.-L. Ng, H. Awala, K.-L. Wong and S. Mintova, Microwave synthesis of colloidal stable AlPO-5 nanocrystals with high water adsorption capacity and unique morphology, Mater. Lett., 2014, 132, 126–129 CrossRef CAS PubMed.
- L. Zhang, X. Chen, S. Jin, J. Guan, C. T. Williams, Z. Peng and C. Liang, Rapid microwaves synthesis of CoSix/CNTs as novel catalytic materials for hydrogenation of phthalic anhydride, J. Solid State Chem., 2014, 217, 105–112 CrossRef CAS PubMed.
- K. Ullah, S. Ye, Z. Lei, K. Y. Cho and W. C. Oh, Synergistic effect of PtSe2 and graphene sheets supported by TiO2 as cocatalysts synthesized via microwave techniques for improved photocatalytic activity, Catal. Sci. Technol., 2015, 5, 184–198 CAS.
- L. Gu, L. Qian, Y. Lei, Y. Wang, J. Li, H. Yuan and D. Xiao, Microwave-assisted synthesis of nano sphere-like NiCo2O4 consisting of porous nanosheets and its application in electro-catalytic oxidation of methanol, J. Power Sources, 2014, 261, 317–323 CrossRef CAS PubMed.
- K. Ullah, S. Ye, L. Zhu, Z. D. Meng, S. Sarkar and W. C. Oh, Microwave assisted synthesis of a noble metal–graphene hybrid photocatalyst for high efficient decomposition of organic dyes under visible light, Mater. Sci. Eng., B, 2014, 180, 20–26 CrossRef CAS PubMed.
- S. D. Perera, R. G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal and K. J. Balkus Jr, Hydrothermal synthesis of graphene–TiO2 nanotube composites with enhanced photocatalytic activity, ACS Catal., 2012, 2, 949–956 CrossRef CAS.
- B. Pejova, Optical phonons in nanostructured thin films composed by zinc blende zinc selenide quantum dots in strong size-quantization regime: Competition between phonon confinement and strain-related effects, J. Solid State Chem., 2014, 213, 22–31 CrossRef CAS PubMed.
- B. D. Cullity, Elements of X-rays Diffraction, Addison Wesley Publishing Co, Philippines, 2nd edn, 1978, ch. 10, p. 338 Search PubMed.
- E. Anastassakis, Light scattering in transition metal diselenide CoSe2 and CuSe2, Solid State Communications, vol. 13, 1973, pp. 1297–1301 Search PubMed.
- L. Zhu, M. Teo, P. C. Wong, K. C. Wong, I. Narita, F. Ernst, K. A. R. Mitchell and S. A. Campbell, Synthesis, characterization of a CoSe2 catalyst for the oxygen reduction reaction, Appl. Catal., A, 2010, 386, 157–165 CrossRef CAS PubMed.
- A. Das, S. Pisana, B. Chakraborty, S. Piscanec, S. K. Saha and U. V. Waghmare, et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor, Nat. Nanotechnol., 2008, 3, 210–215 CrossRef CAS PubMed.
- Z. H. Ni, T. Yu, Z. Q. Luo, Y. Y. Wang, L. Liu and C. P. Wong, et al. Probing charged impurities in suspended graphene using Raman spectroscopy, ACS Nano, 2009, 3(3), 569–574 CrossRef CAS PubMed.
- Z. H. Ni, T. Yu, Y. H. Lu, Y. Y. Wang, Y. P. Feng and Z. X. Shen, Uniaxial strain on graphene: Raman spectroscopy study and bandgap opening, ACS Nano, 2008, 2(11), 2301–2305 CrossRef CAS PubMed.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets, Nanoscale, 2011, 3, 3670–3678 RSC.
- G. M. Zhou, D. W. Wang, L. C. Yin, N. Li, F. Li and H. M. Cheng, Oxygen Bridges between NiO Nanosheets and Graphene for Improvement of Lithium Storage, ACS Nano, 2012, 6, 3214–3223 CrossRef CAS PubMed.
- D. Kong, H. Wang, Z. Lu and Y. Cui, CoSe2 Nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction, J. Am. Chem. Soc., 2014, 136(13), 4897–4900 CrossRef CAS PubMed.
- J. S. Lee, K. H. You and C. B. Park, Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene, Adv. Mater., 2012, 24, 1084–1088 CrossRef CAS PubMed.
- R. Rao, R. Podila, R. Tsuchikawa, J. Katoch, D. Tishler, A. Rao and I. M. shigami, Effects of Layer Stacking on the Combination Raman Modes in Graphene, ACS Nano, 2011, 5(3), 1594–1599 CrossRef CAS PubMed.
- K. John, D. T. Manolis, D. P. George, N. A. Mariza, S. T. Kostas, G. Sofia, B. Kyriakos, K. Christos, O. Michael and L. Alexis, Highly active catalysts for the photo oxidation of organic compounds by deposition of fullerene onto the MCM-41 surface: a green approach for the synthesis of fine chemicals, Appl. Catal., B, 2012, 117–118, 36–48 Search PubMed.
- K. Ullah, S. Ye, L. Zhu, S. B. Jo, W. K. Jang and W. C. Oh, Noble metal doped graphene nanocomposites and its study of photocatalytic hydrogen evolution, Solid State Sci., 2014, 31, 91–98 CrossRef CAS PubMed.
- G. Xie, K. Zhang, B. Guo, Q. Liu, L. Fang and J. R. Gong, Graphene-based materials for hydrogen generation from light-driven water splitting, Adv. Mater., 2013, 25, 3820–3839 CrossRef CAS PubMed.
- P. V. Kamat, Graphene-based nano architectures: anchoring semiconductor and metal nanoparticles on a two dimensional carbon support, J. Phys. Chem. Lett., 2010, 1, 520–527 CrossRef CAS.
- P. Hu, Y. D. Posner, Y. B. David and D. Milstein, Reusable homogeneous catalytic system for hydrogen production from methanol and water, ACS Catal., 2014, 4(8), 2649–2652 CrossRef CAS.
- B. J. Ma, J. S. Kim, C. H. Choi and S. I. Woo, Enhanced hydrogen generation from methanol aqueous solutions over Pt/MoO3/TiO2 under ultraviolet light, Int. J. Hydrogen Energy, 2013, 38, 3582–3587 CrossRef CAS PubMed.
- K. Ullah, A. Ali, S. Ye, L. Zhu and W. C. Oh, Microwave-assisted synthesis of Pt–graphene/TiO2 nanocomposites and their efficiency in assisting hydrogen evolution from water in the presence of sacrificial agents, Sci. Adv. Mater., 2015, 7(4), 606–614 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |