Catalytic properties and deactivation behavior of H-MCM-22 in the conversion of methanol to hydrocarbons†
Pengfei Wangab,
Lizhi Huanga,
Junfen Lia,
Mei Donga,
Jianguo Wanga,
Takashi Tatsumic and
Weibin Fan*a
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China. E-mail: fanwb@sxicc.ac.cn; Fax: +86-351-4041153; Tel: +86-351-4199009
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cChemical Resources Laboratory, Tokyo Institute of Technology, Nagatsuta 4259, Midori-ku, Yokohama 226-8503, Japan
First published on 17th March 2015
Abstract
The catalytic properties of H-MCM-22 zeolites with Si/Al ratios in the range from 13.5 to 47 and their dealuminated samples were investigated in the conversion of methanol to hydrocarbons. The fresh and spent samples were characterized with XRD, NH3-TPD, pyridine-adsorption infrared spectroscopy, 27Al MAS NMR spectroscopy, TG-DTA, GC-MS, diffuse reflectance UV-vis, Raman spectroscopy techniques and nitrogen and p-xylene adsorption experiments. Although a decrease in protonic acid sites, as expected, increased the selectivity to light olefins such as propene and butene, the sample with a Si/Al ratio of 25–37 showed higher catalytic stability. It is worth noting that the external surface acid sites have a pronounced effect on the catalytic performance. Selective removal of these acid sites by dealumination with oxalic acid markedly increased the catalytic stability. This is because the acid sites in the supercages and external side pockets quickly induced formation of deposited coke species, which blocked the sinusoidal channels and caused the deactivation of the catalyst.
1. Introduction
The conversion of methanol to hydrocarbons (MTH) over acidic zeolites has drawn considerable attention since its discovery in 1970s by Mobil Corporation. This process is considered as a promising alternative route for the production of light olefins, gasoline and aromatics from petroleum because methanol as a platform compound can be readily produced from coal, natural gas and biomass via gas synthesis (CO + H2).1 Depending on the product selectivity, this process was named as MTG (methanol to gasoline), MTO (methanol to olefins), MTP (methanol to propene) and MTA (methanol to aromatics), respectively.2 In the last 40 years, a significant progress has been achieved at both the reaction mechanism and the catalytic process. MTO, MTP and MTG have been industrialized with SAPO-34 or ZSM-5 as catalyst. Regardless of this, the catalytic life of SAPO-34 is shorter than 12 h,3 and hence, it needs fluidized bed for frequent regeneration.4 As for ZSM-5 (Si/Al = 100), the selectivity to ethene (8.1%) and propene (33.1%) is not high although it is highly dependent on the framework Si/Al ratio.5 Therefore, it is still necessary to develop a new zeolite catalyst for increasing both the product selectivity and the catalytic stability despite of possible existence of a balance between them.According to the dual-cycle mechanism, two pathways exist for forming alkenes.6,7 The reaction is initiated by aromatic species occluded in the channel to generate ethene and propene via “side chain” or “paring” routes.8,9 Then, methylation and cracking of light olefins are started to simultaneously produce different types of alkenes.10 These alkenes are further cyclized to form aromatics and alkanes via hydride transfer reaction. The extent for the occurrence of these reactions depends on the zeolite pore structure, acidity and morphology, which determine the catalytic activity, stability and product selectivity. An increasing demand of propene makes the MTH selectivity toward propene attractive. SAPO-34 produces mainly ethene and propene but with a propene to ethene (P/E) ratio of about 1.11,12 Even for ZSM-5, the P/E ratio in the product is usually smaller than 8.5,13,14 Although increase of the zeolite pore openings to 12-membered ring (MR) can significantly increase the P/E ratio, the catalyst rapidly deactivated.14,15
MCM-22 zeolite with 10 MR pore openings has a unique lamellar structure consisting of two independent pore systems.16 One has a large cylindrical supercage (7.1 Å (D) × 18.2 Å (H)) between layers, the other is a two-dimensional (2D) sinusoidal channel within layer. This pore structure gives rise to H-MCM-22 with potential catalytic properties in cracking, isomerization, alkylation and disproportionation.17–21 Recently, MWW-type zeolites have been used in MTH process. Bjørgen and coworkers reported that polymethylated benzenes are readily formed as the dominant reaction intermediates,22 showing the occurrence of aromatics-based hydrocarbon-pool (HP) mechanism probably in the supercages. This is supported by the assumption that the supercages primarily deactivated during the unsteady stage, while the acid sites located in the sinusoidal channel and on the surface pocket dominated the MTH process.23 Thus, the catalytic stability and propene selectivity of MCM-22 was increased by selective dealumination of its surface pockets.24 This suggests that the framework Al distribution in H-MCM-22 has a pronounced effect on its catalytic properties. Indeed, it was found that different reactions occurred in the different pore systems. The catalytic cracking of n-heptane18 and isomerization of m-xylene19 mainly occur in the sinusoidal channels, while the disproportionation of toluene to p-xylene is related to the large supercages.20 For the liquid-phase alkylation of benzene with ethene and propene, they mainly take place in the side pockets on the external surface, giving high selectivity to ethylbenzene and cumene respectively.21
Thus, the MTH catalytic properties of hydrothermally synthesized and acid-dealuminated H-MCM-22 (Si/Al = 13.5–47) were systematically studied here to investigate the effects of acid site amount and distribution. In addition, the deactivation behavior of MCM-22 in the MTH process was also investigated. It is shown that the acid sites in the supercages and the sinusoidal channels play a different role in catalyzing the MTH process, and the olefin methylation-cracking mechanism dominates the process despite that the HP mechanism initiates the reaction in the supercages.
2. Experimental
2.1 Sample preparation
A series of MCM-22 samples were hydrothermally synthesized with silica sol (40.5 wt% of SiO2, Qingdao Haiyang Chem. Co., Ltd.), sodium aluminate (41 wt% of Al2O3, 41 wt% of Na2O, Sinopharm Chem. Reagent Co., Ltd.), hexamethyleneimine (HMI, 98 wt%, Jiangsu Fengyuan Bioengineering Co., Ltd.), NaOH (96 wt%, Sinopharm Chem. Reagent Co., Ltd.) and deionized water according to the reported procedures.25 The molar ratio of the synthesis gels was as follows: 0.18 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 : xAl2O3 : 0.35 HMI
SiO2 : xAl2O3 : 0.35 HMI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 H2O, where x = 0.0333, 0.02, 0.0135 and 0.01. For example, when x was 0.02, 0.607 g of NaOH and 0.445 g of NaAlO2 were first dissolved in 73 g of deionized water and stirred for 10 min. Then, 3.50 g HMI was introduced under stirring conditions. Finally, 15.0 g silica sol was dropwise added and further stirred for 2 h. The resultant gel was crystallized in a rotating (30 rpm) Teflon-lined stainless-steel autoclave at 150 °C for 7d. The products were filtered, washed and dried overnight at 100 °C, and further calcined at 560 °C for 10 h in air. The obtained Na-MCM-22 samples were repeatedly ion-exchanged with NH4NO3 aqueous solution (1 M) at 80 °C for 6 h. This is followed by calcination at 540 °C for 6 h to form H-MCM-22.
45 H2O, where x = 0.0333, 0.02, 0.0135 and 0.01. For example, when x was 0.02, 0.607 g of NaOH and 0.445 g of NaAlO2 were first dissolved in 73 g of deionized water and stirred for 10 min. Then, 3.50 g HMI was introduced under stirring conditions. Finally, 15.0 g silica sol was dropwise added and further stirred for 2 h. The resultant gel was crystallized in a rotating (30 rpm) Teflon-lined stainless-steel autoclave at 150 °C for 7d. The products were filtered, washed and dried overnight at 100 °C, and further calcined at 560 °C for 10 h in air. The obtained Na-MCM-22 samples were repeatedly ion-exchanged with NH4NO3 aqueous solution (1 M) at 80 °C for 6 h. This is followed by calcination at 540 °C for 6 h to form H-MCM-22.
The obtained H-MCM-22 sample was treated with 1 M or 0.5 M oxalic acid solution at 80 °C for 10 h under reflux conditions. Then, it was filtered, washed with distilled water, dried at 100 °C overnight and calcined at 500 °C for 4 h. The parent and oxalic acid-treated samples were designated as H-MCM-22-x and H-MCM-22-x-o respectively.
2.2 Sample characterization
The H-MCM-22 structure was confirmed by its X-ray diffraction (XRD) pattern recorded on a Rigaku MiniFlexII X-ray diffractometer with CuKα radiation at a scanning speed of 1°/min. Nitrogen adsorption/desorption isotherms of samples were measured at −196 °C on a Belsorp-max instrument. Prior to the measurement, the fresh and coked samples were pretreated at 300 °C and 150 °C for 5 h respectively under high vacuum conditions. The specific surface area and the micropore volume were calculated by the BET and the t-plot methods respectively. The bulk chemical compositions were determined by an inductively coupled plasma-atomic emission spectrometer (ICP-AES, iCAP6000).The temperature-programmed desorption of ammonia (NH3-TPD) were carried out on a Micromeritics Autochem II 2920 instrument equipped with a thermal conductivity detector (TCD). 100 mg of sample was first pretreated at 550 °C for 1 h in a He flow (30 ml min−1). After being cooled to 120 °C, it was purged with a 10% NH3–He flow for 30 min. Then, it was flushed with a pure He flow at 120 °C for 2 h to remove physically adsorbed ammonia. Finally, it was heated to 550 °C in the He flow at a rate of 10 °C min−1, and the desorbed ammonia was monitored with a TCD.
The OH-region and pyridine-adsorbing IR (Py-IR) spectra of H-MCM-22 were measured on a Bruker Tensor 27 spectrometer at a resolution of 4 cm−1. Before measuring the spectra, a self-supporting sample wafer (15 mg) was prepared and evacuated at 450 °C and 2 × 10−2 Pa for 2 h. The OH-region IR spectra were recorded by accumulating 64 scans after the sample was cooled to room temperature. The Py-IR spectra were measured after exposing the pretreated wafer to pyridine vapor for 30 min at room temperature (RT) and subsequently outgassing at 150 or 350 °C and 10−2 Pa for 30 min. The amounts of Brönsted and Lewis acid sites were calculated with the extinction coefficients of 1.13 and 1.28 cm μmol−1 respectively according to the method reported in the ref. 26.
27Al MAS NMR spectra of samples were measured on a 600 MHz Bruker Advance III nuclear magnetic resonance spectrometer equipped with a 4 mm probe at a spinning rate of 13 kHz. The spectra were recorded using one pulse sequence with a π/12 pulse width of 1.0 μs and a 2 s recycle delay. The chemical shifts were referenced to Al(NO3)3 aqueous solution. The ratio of framework to extra-framework aluminum species (AlIV/AlVI) was determined by integrating the area of the signal around 56 ppm to that of the signal at 0 ppm.
The content of deposited coke in the sample was determined by a Rigaku Thermo Plus Evo 8120 thermogravimetric instrument. The coked sample was heated from RT to 800 °C in air at a rate of 10 °C min−1. The diffuse reflectance (DR) UV-visible spectra were measured in the range of 200–800 nm on a Shimadzu UV-vis spectrophotometer (UV-3600). The soluble carbonaceous species in the deposited coke was extracted with methylene chloride (CH2Cl2) after dissolving the sample in hydrofluoric (HF) acid and analyzed by a Shimadzu gas chromatograph-mass spectrometer (ultra plus GC-MS) equipped with a Rtx-5MS capillary column. Raman spectra of coked samples were measured on a Horiba Labram HP800 spectrometer using the 514 nm incident light of the Ar+ laser.
2.3 Catalytic test
The MTH reaction was carried out in a fixed-bed reactor (i.d. 10 mm) loaded with 1.0 g catalyst (0.35–0.83 mm). The catalyst was activated at 550 °C for 2 h in a N2 flow before the reaction. The typical reaction conditions are as follows: WHSVmethanol of 2 h−1, 450 °C, atmospheric pressure, N2 as balance gas (40 mL min−1). The gas product was on-line analyzed on an Angilent 7890A gas chromatograph equipped with DB-1, OxyPlot and Al2O3/KCl plot columns and two flame ionization detectors (FID) and one TCD. The liquid products were separated into oil and aqueous phases, which were analyzed by another two Angilent 7890A gas chromatographs equipped with a HP-PONA or a HP-INNOWAX column and a FID. It should be noted that dimethyl ether (DME) was considered as unconverted methanol when calculating methanol conversion.3. Results and discussion
3.1 Catalyst characterization
Fig. 1 shows that all the parent H-MCM-22 and the oxalic acid-treated samples have a typical MWW structure.25,27 This shows that H-MCM-22 with Si/Al ratios in the range from 13.5 to 47 can be synthesized with a high crystallinity (Table 1), as supported by the N2 sorption measurement that all the samples have high surface area and pore volume. Nonetheless, the intensity of diffraction lines still depends on the Si/Al ratio in the synthesis gel, revealing different crystallinities of the synthesized H-MCM-22. Regardless of this, dealumination of the H-MCM-22 with designed concentration of oxalic acid does not have great effects on the crystalline structure. This is confirmed by the nitrogen physisorption results that no significant changes in the textural properties occurred during the oxalic acid treatment (Fig. 1 and Table 1).| Sample | Si/Al | SBET (m2 g−1) | Vmic (cm3 g−1) | Acid sites (mmol/g) | c (μmol g−1) 150 °C | c (μmol g−1) 350 °C | (AlF/AlEF) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weak | Strong | B | L | B/L | B | L | B/L | |||||
| H-MCM-22-15 | 13.5 | 524 | 0.188 | 0.64 | 0.42 | 317 | 270 | 1.2 | 236 | 142 | 1.7 | 82/18 |
| H-MCM-22-15-o | 22.1 | 468 | 0.169 | 0.33 | 0.34 | 209 | 162 | 1.3 | 165 | 73 | 2.3 | 81/19 |
| H-MCM-22-25 | 23.6 | 492 | 0.182 | 0.35 | 0.39 | 330 | 138 | 2.4 | 233 | 84 | 2.8 | 87/13 |
| H-MCM-22-25-o | 31.5 | 464 | 0.176 | 0.24 | 0.33 | 179 | 89 | 2.0 | 148 | 38 | 3.9 | 88/12 |
| H-MCM-22-37 | 35.7 | 513 | 0.195 | 0.21 | 0.27 | 172 | 90 | 1.9 | 157 | 43 | 3.7 | 91/9 |
| H-MCM-22-37-o | 44.8 | 518 | 0.192 | 0.14 | 0.26 | 155 | 65 | 2.4 | 136 | 23 | 5.8 | 92/8 |
| H-MCM-22-50 | 47.0 | 466 | 0.178 | 0.13 | 0.24 | 157 | 68 | 2.3 | 122 | 38 | 3.2 | 94/6 |
The NH3-TPD profiles (Fig. S1†) of all the H-MCM-22 catalysts are characterized by two distinct desorption peaks around 195 and 360 °C, which are assigned to desorption of NH3 from weak and strong acid sites, respectively. It should be pointed out that the slight shift of these two desorption peaks to high temperatures with the framework Al content may be due to the increase in the acid amount, not acid strength.27 The 10 MR pore openings of H-MCM-22 make oxalic acid mainly remove its Al species near the pore mouth and on the external surface.24 Thus, the Al species in the side pockets would be mostly washed out by the oxalic acid, while those in the interior void space are difficult to be removed. This is supported by the result that more weak acid sites were removed than strong ones by the oxalic acid treatment (Table 1).
Fig. 2A shows that two intense bands are present at 3745 and 3620 cm−1 in the OH-region IR spectra of all the H-MCM-22 catalysts. In addition, three weak bands were also observed around 3730, 3693 and 3670 cm−1. The band at 3745 cm−1 is generally ascribed to free terminal Si–OH groups located on the external surface, while its shoulder at 3730 cm−1 is attributed to the internal silanol groups. After oxalic acid treatment, the band at 3745 cm−1 increased in the intensity, indicative of a significant dealumination on the external surface.
 | ||
| Fig. 2 OH-region IR (A) and Py-IR spectra (B) of (a) H-MCM-22-15, (b) H-MCM-22-15-o, (c) H-MCM-22-25, (d) H-MCM-22-25-o, (e) H-MCM-22-37, (f) H-MCM-22-37-o and (g) H-MCM-22-50. | ||
The intense band at 3620 cm−1 and its shoulder band at 3580 cm−1 are due to the bridging Si–OH–Al groups with Al located at crystallographically inequivalent sites.28–30 It seems that the 3620 cm−1 band becomes broader with the Al amount in the framework (Fig. 2a, c, e and g). This may be because Al occupied more T sites when more Al species were incorporated in the framework. It is noteworthy that the band at 3670 cm−1, attributed to Al–OH species,31 decreased in the intensity with increasing Si/Al ratio in the samples, indicating a decrease in the amount of extra-framework Al species. As expected, the oxalic acid treatment also decreased the amount of extra-framework Al species. The presence of a broad band at 3510 cm−1 originating from the OH nests was found in the IR spectra of all the oxalic acid-treated samples due to the dealumination, which led to formation of considerable amounts of defect sites.
The Py-IR spectra measured after desorption of pyridine at 150 °C of all the H-MCM-22 catalysts are shown in Fig. 2B. All the H-MCM-22 samples possess one type of Brönsted acid site (1543 and 1635 cm−1) and two types of Lewis acid sites (1453 and 1620, and 1445 and 1610 cm−1), and both the Brönsted and Lewis acid sites amounts increased with increasing Al contents in the samples (Table 1). Although the oxalic acid treatment removed considerable amounts of Brönsted and Lewis acid sites, it is unexpected that much more strong Lewis acid sites were eliminated than strong Brönsted acid sites while it is not obvious for the weak acid sites. This is perhaps because of the easier removal of extra-framework Al species by oxalic acid treatment.
Fig. 3A shows the 27Al MAS NMR spectra of all the H-MCM-22 catalysts. Clearly, all the samples contain tetrahedral (AlIV, the broad signal around 56 ppm) and octahedral (AlVI, the signal at 0 ppm) Al species,29,32,33 and the amount of AlVI species decreased with increasing Si/Al ratio in the H-MCM-22 (Table 1). This is in agreement with the above-obtained results with IR spectroscopy that the amount of extra-framework Al species decreased with the Si/Al ratio. The broad signal around 56 ppm contains at least three components centered at 50.5, 56.0 and 61.5 ppm, which are attributed to the Al species located at crystallographically different T sites.34 It has been reported that MCM-22 zeolite has eight crystallographically inequivalent T sites,16 as shown in Scheme S1.† These three components were tentatively assigned to Al sited at T6 and T7 (50.5 ppm), T8, T5, T4, T1 and T3 (56.0 ppm), and T2 (61.5 ppm) sites, respectively.35 Recently, the broad signal was resolved into five fine components.33 The significant difference in the relative intensity of the five components of H-MCM-22-15 and H-MCM-22-50 indicates that the Al distribution in the framework depends on its content in the sample (Fig. S2†). It was reported that the Al species at 50.5 ppm disappeared after the oxalic acid treatment,36 which, however, was not observed in our experiment. In particular, no significant change in the relative intensity occurred to the three components around 50.5, 56.0 and 61.5 ppm. In addition, the AlIV/AlVI ratio of dealuminated sample was similar to the parent one, showing that the framework and extraframework Al species were proportionally removed (Table 1).
Fig. 3A(c) shows that a certain amount of octahedral Al species are present in the H-MCM-22-25. However, it was not detected in the NH4-MCM-22-25 (Fig. 3B(a)). This shows that a part of AlIV species transformed into AlVI during the deammoniation process. The same phenomenon was observed for the oxalic acid-treated sample (Fig. 3B(b) and B(c)), showing that AlVI species in both the H-MCM-22 and the oxalic acid dealuminated samples can change to tetrahedral coordination upon adsorption of NH3. This reversible coordination change indicates that these AlVI species may be partially bound to framework silicon atoms via oxygen bridges.37
3.2 Catalytic properties and deactivation behavior
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) /C2
/C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ratio was observed in the products obtained over high-silica H-MCM-22 and H-MCM-22-o (Fig. 5F). It should be noted that at the initial stage, the ethene selectivity significantly decreased with the reaction time for all the samples.
ratio was observed in the products obtained over high-silica H-MCM-22 and H-MCM-22-o (Fig. 5F). It should be noted that at the initial stage, the ethene selectivity significantly decreased with the reaction time for all the samples.
In the beginning of the MTH reaction, C4-HTI was about 0.9 for all the H-MCM-22 catalysts, but it sharply decreased to <0.5 within 3 h, and a more pronounced decrease was observed for the oxalic acid-dealuminated samples than for the hydrothermally synthesized samples. This indicates that although large amounts of aliphatic alkanes and aromatics are formed in the early stage,22 their amounts quickly decrease with increasing reaction time, as shown in Fig. S3,† for both the parent and the oxalic acid-dealuminated H-MCM-22 catalysts, and the dealuminated sample shows lower selectivity to alkanes and aromatics than the parent one due to decrease of hydrogen transfer reaction. It is worth noting that no CO and CO2 were detected in the whole MTH process.
Fig. 5E shows that the methane selectivity decreased with the reaction time within the first 2 h for all the samples. However, it unexpectedly increased depending on the Si/Al ratio when the reaction time was longer than 2 h although the catalysts did not deactivate. A steep increase was observed over the H-MCM-22-15 and the H-MCM-22-25 catalysts, while the samples with lower amounts of Al or acid sites showed a slow increase before the deactivation, and a more slowly increase in the methane selectivity occurred after selectively washing the acid sites with oxalic acid even if the catalyst had a high content of framework Al species. It was suggested that methane was generated by methylation of the coke with methanol.38 It can be used as a measure of catalyst deactivation in MTH reaction.24 High methane selectivity indicates deposition of a large amount of coke on the catalyst surface, causing the catalyst deactivation. Thus, the slow increase in the methane selectivity gives another piece of evidence for the high catalytic stability of the oxalic acid-treated samples.
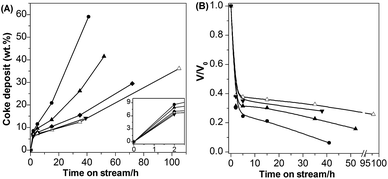
It is worth noting that all the catalysts decreased by about 65% in the pore volume within 2 h (Fig. 6B), showing that about 2/3 of void space in the framework was occupied or blocked. This indicates that the coking mainly occurred in the supercages since it has been measured that around 63% of the total micropore volume is due to the supercages and another 32% results from the sinusoidal channels.39 This is supported by the p-xylene adsorption result at 25 °C that the amount adsorbed on the catalyst reacted for 2 h was about 1/3 of that on the fresh sample (Fig. S5†). Molecular simulation of p-xylene adsorption on ITQ-1 structurally analogous to MCM-22 indicates that there are three molecules per unit cell; one sites in the sinusoidal channels, and the other two are located at the supercages.40 The quick deactivation of supercages was also observed in butene, n-heptane cracking and m-xylene transformation.19,41 This is understandable because the large supercages allow formation of bulky coke precursors, which would be trapped inside it as a result of the diffusion limit by its 10 MR pore-opening.42 Irrespective of this, the ratios of the volume occupied by coke (VR) to that inaccessible to the adsorbate (VA) of H-MCM-22-15 and H-MCM-22-25-o are close to 0.54 and 0.52 respectively at the reaction time of 2 h, as estimated by assuming the coke density of about 1.2 g cm−3. This shows that the deactivation of the supercages is not due to the void filling but the pore blockage by coke since the VR/VA is much lower than 1.43
The above results suggest that the deactivation of H-MCM-22 should be caused by the coke deposited on the external surface since there are many side pockets (hemicages). This is supported by the finding that the VR/VA ratio increased to the value much larger than 1 after reaction of 2 h. As mentioned above, the oxalic acid treatment mainly removed the external surface acid sites, but it sharply decreased the coking rate and increased the catalytic stability, showing that the coke on the external surface side pockets indeed caused the deactivation of H-MCM-22.
Nonetheless, Fig. 7A shows that both the 3620 cm−1 and the 3747 cm−1 bands greatly decreased in the intensity, and even disappeared with increasing reaction time for all the H-MCM-22 catalysts. This shows that the deposited coke also severely covered the acid sites, including external surface weak acid sites. This is supported by the significant decrease of the band at 1545 cm−1 in the intensity after adsorption of pyridine (Fig. 7B). The much smaller decreased degrees of the oxalic acid-treated samples such as H-MCM-22-25-o than those of the parent ones (spectra e vs. f) give another piece of evidence for their high catalytic stability.
It is unexpected that a broad negative absorbance band was observed between 1560 and 1610 cm−1 in the spectra of coked catalysts after adsorption of pyridine. This may be caused by the substitution of deposited coke molecules by basic pyridine.44 At the reaction time of 2 h, the Brönsted acid site amounts in the H-MCM-22-15, H-MCM-22-25 and H-MCM-22-25-o were estimated to be 206, 193 and 119 μmol g−1, which are about 65%, 58% and 66% of those of the fresh samples, respectively. However, the above nitrogen sorption measurements revealed that about 65% of micropore volume (Fig. 6B), corresponding to the void space of supercages, was blocked at this reaction time, and about 50–70% of bridging hydroxyl groups was estimated to locate in the supercages and the other 20–30% is in the sinusoidal channels.30 This suggests that the substitution of pyridine for coke molecules should mainly occur at the entrance of supercages in the early stage. Nevertheless, a further increase in the reaction time to 15 h resulted in a drastic reduction in the amounts of Brönsted acid sites accessible to pyridine molecules to 25 and 82 μmol g−1 for H-MCM-22-15 and H-MCM-22-25, respectively. In contrast, 116 μmol of Brönsted acid sites was still detected in one gram of H-MCM-22-25-o, indicative of a slow coking rate.
The DR UV-vis spectroscopy is able to estimate the unsaturation degree and aromaticity of deposited coke.47 At the reaction time of 2 h, all the catalysts show 9 absorbance bands at 215, 250, 310, 345, 385, 450, 540, 640 and 760 nm (Fig. 9A). Although the assignment of all of these bands is not straightforward, the bands at 310, 385, 450 and 540 nm were ascribed to monoenic, dienic, trienic and tetraenic allylic cations,47 while those at 260, 340 and 410 nm were supposed to be due to the polyaromatics composed of two-, three- and four-benzene fused rings, respectively.48 The presence of broad absorbance bands around 640 and 760 nm may be caused by the condensed aromatic rings with more extended conjugated hydrocarbons.49 The formation of these unsaturated carboncations could be reasonable because consecutive hydrogen transfer reactions from the olefins occur along with the generation of alkanes. As the reaction goes on, the 540 nm band increases in intensity at the expense of the band at 450 nm (Fig. 9B). This indicates that the initially formed monoenic species transform into more highly conjugated species,50 and further to highly polymerized aromatic compounds.
 | ||
| Fig. 9 DR UV-vis spectra of (a) H-MCM-22-15, (b) H-MCM-22-25-o and (c) H-MCM-22-37 at the reaction time of 2 h (A) and 15 h (B), and of the extracted coke species with CH2Cl2 (C). | ||
In order to more clearly identify the coking species, the spent catalysts were dissolved with 10% of HF aqueous solution, and the soluble coking species was extracted with CH2Cl2. Fig. 9C shows the UV-vis spectra of the CH2Cl2-extracted coking species. Only the bands below 500 nm are discernible. It is clear that the bands at 250, 310, 345 and 430 nm decrease in intensity with the acid amount in the samples and the reaction time, further proving the transformation of monoenic species into the fused-ring aromatics since the amounts of deposited coke and insoluble coke species both increase. This is more clearly shown by the GC-MS analysis result. The soluble deposited coke species mainly contains benzene, methylbenzene, multimethylbenzenes, naphthalene, methylnaphthalene and fused-ring aromatics (Fig. S6†). In addition, alcohols, ketones and long-chain (>C15) alkanes were also detected. Apparently, the amounts of the substances with molecules larger than naphthalene increased with increasing reaction time. The insoluble black coke was identified by Raman spectroscopy. Two peaks were observed at 1340 and 1600 cm−1 (Fig. S7†), which can be assigned to the graphite-like carbonaceous species.51
Dealumination of the H-MCM-22 with oxalic acid selectively removed both the Brönsted and Lewis acid sites on the external surface and near the pore mouth, while those in the sinusoidal channels are retained. This not only hinders the conversion of propene and butene into bulky aromatics (coke precursors), but also decreases the hydrogen transfer reaction. Therefore, higher propene and butene selectivity and catalytic stability was obtained over the oxalic acid-dealuminated sample. Although an increase in the Si/Al ratio decreases the acid sites, and hence, suppressing the secondary reaction and enhancing the propene and butene selectivity, the catalytic stability was lowered down probably because incorporation of more Al in the sinusoidal channels are more difficult than in the supercages,30 making the amount of the acid sites located in the sinusoidal channels smaller.
4. Conclusions
A series of highly crystalline MCM-22 sample with Si/Al ratios from 13.5 to 47 was synthesized. The amount of octahedral Al species increased with decreasing Si/Al ratio in the sample. Upon adsorption of ammonia, these octahedral Al species were transformed into tetrahedral Al species, showing that they are tri-coordinated to framework. The MTH catalytic properties of H-MCM-22 are highly dependent on its Al content or acid amount. An increase in the Si/Al ratio leads to an increase in propene and butene selectivity but a decrease in ethene selectivity. The sample with a Si/Al ratio of 35.7 exhibits much higher catalytic stability than the other samples because of its suitable acid site density and distribution. Nonetheless, all the samples show similar coking rate within initial 2 h, which causes the deactivation of the supercages. This is substantiated by the result that a selective removal of the acid sites in the side pockets markedly increased the catalytic stability. Despite that H-MCM-22-25-o and H-MCM-22-37-o show higher propene selectivity and P/E ratio, H-MCM-22-15-o is much more stable, showing that the catalytic stability of the oxalic acid-dealuminated sample increases with increasing acid sites in the sample under employed conditions. The deposited coke species mainly consists of mutimethyl benzene, naphthalene, methylnaphthalene and fused-ring aromatics, which are formed from monoenic species. With increasing reaction time, these species transform into graphite-like coke, and the transformation process is sped up by increasing acid amounts in the sample. Although the MTH process is initiated by the aromatics-based HP mechanism over the H-MCM-22, the reaction primarily occurs in the 10 MR sinusoidal channel in the steady stage, and thus, the olefins methylation and cracking mechanism dominates the process, resulting in a drastic increase in the selectivity to propene and butene but a sharp decrease in the selectivity to ethene within a few of hours.Acknowledgements
This work is supported by the National Basic Research Program (nos 2010CB234603, 2011CB201403 and 2011CB201406) and the National Natural Science Foundation of China (nos 10979068, 21003148 and 21273264).References
- U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS PubMed.
- J. Q. Chen, A. Bozzano, B. Glover, T. Fuglerud and S. Kvisle, Catal. Today, 2005, 106, 103–107 CrossRef CAS PubMed.
- Y.-J. Lee, S.-C. Baek and K.-W. Jun, Appl. Catal., A, 2007, 329, 130–136 CrossRef CAS PubMed.
- P. Tian, Y. Wei, M. Ye and Z. Liu, ACS Catal., 2015, 5, 1922–1938 CrossRef CAS.
- J. Liu, C. Zhang, Z. Shen, W. Hua, Y. Tang, W. Shen, Y. Yue and H. Xu, Catal. Commun., 2009, 10, 1506–1509 CrossRef CAS PubMed.
- S. Svelle, F. Joense, J. Nerlov, U. Olsbye, K.-P. Lillerud, S. Kolboe and M. Bjørgen, J. Am. Chem. Soc., 2006, 128, 14770–14771 CrossRef CAS PubMed.
- M. Bjørgen, S. Svelle, F. Joensen, J. Nerlov, S. Kolboe, F. Bonino, L. Palumbo, S. Bordiga and U. Olsbye, J. Catal., 2007, 249, 195–207 CrossRef PubMed.
- B. Arstad and S. Kolboe, J. Am. Chem. Soc., 2001, 123, 8137–8138 CrossRef CAS.
- W. Song, H. Fu and J. F. Haw, J. Am. Chem. Soc., 2001, 123, 4749–4754 CrossRef CAS PubMed.
- R. M. Dessau and R. B. Lapierre, J. Catal., 1982, 78, 136–141 CrossRef CAS.
- G. Yang, Y. Wei, S. Xu, J. Chen, J. Li, Z. Liu, J. Yu and R. Xu, J. Phys. Chem. C, 2013, 117, 8214–8222 CAS.
- M. Sedighi, H. Bahrami and J. Towfighi Darian, RSC Adv., 2014, 4, 49762–49769 RSC.
- C. D. Chang, C. T. W. Chu and R. F. Socha, J. Catal., 1984, 86, 289–296 CrossRef CAS.
- S. Park, Y. Watanabe, Y. Nishita, T. Fukuoka, S. Inagaki and Y. Kubota, J. Catal., 2014, 319, 265–273 CrossRef CAS PubMed.
- Ø. Mikkelsen and S. Kolboe, Microporous Mesoporous Mater., 1999, 29, 173–184 CrossRef.
- M. E. Leonowicz, J. A. Lawton, S. L. Lawton and M. K. Rubin, Science, 1994, 264, 1910–1913 CAS.
- R. Ravishankar, D. Bhattacharya, N. E. Jacob and S. Sivasanker, Microporous Mater., 1995, 4, 83–93 CrossRef CAS.
- D. Meloni, D. Martin and M. Guisnet, Appl. Catal., A, 2001, 215, 67–79 CrossRef CAS.
- S. Laforge, D. Martin, J. L. Paillaud and M. Guisnet, J. Catal., 2003, 220, 92–103 CrossRef CAS.
- P. Wu, T. Komatsu and T. Yashima, Microporous Mesoporous Mater., 1998, 22, 343–356 CrossRef CAS.
- A. Corma, V. Martínez Soria and E. Schnoeveld, J. Catal., 2000, 192, 163–173 CrossRef CAS.
- M. Bjørgen, S. Akyalcin, U. Olsbye, S. Benard, S. Svelle and S. Kolboe, J. Catal., 2010, 275, 170–180 CrossRef PubMed.
- A. Lacarriere, F. Luck, D. Świerczyński, F. Fajula and V. Huleaa, Appl. Catal., A, 2011, 402, 208–217 CrossRef CAS PubMed.
- H. K. Min, M. B. Park and S. B. Hong, J. Catal., 2010, 271, 186–194 CrossRef CAS PubMed.
- A. Corma, C. Corell and J. P. Pariente, Zeolites, 1995, 15, 2–8 CrossRef CAS.
- M. Guisnet, P. Ayrault and J. Datka, Pol. J. Chem., 1997, 17, 1445–1454 Search PubMed.
- K. Okumura, M. Hashimoto, T. Mimura and M. Niwa, J. Catal., 2002, 206, 23–28 CrossRef CAS.
- B. Onida, F. Geobaldo, F. Testa, F. Crea and E. Garrone, Microporous Mesoporous Mater., 1999, 30, 119–127 CrossRef CAS.
- A. Corma, C. Corell, V. Fornés, W. Kolodziejski and J. P. Pariente, Zeolites, 1995, 15, 576–582 CrossRef CAS.
- D. Meloni, S. Laforge, D. Martin, M. Guisnet, E. Rombi and V. Solinas, Appl. Catal., A, 2001, 215, 55–66 CrossRef CAS.
- B. Onida, F. Geobaldo, F. Testa, R. Aiello and E. Garrone, J. Phys. Chem. B, 2002, 106, 1684–1690 CrossRef CAS.
- D. Ma, F. Deng, R. Fu, X. Han and x. Bao, J. Phys. Chem. B, 2001, 105, 1770–1779 CrossRef CAS.
- M. Hunger, S. Ernst and J. Weitkamp, Zeolites, 1995, 15, 188–192 CrossRef CAS.
- E. Lippmaa, A. Samoson and M. Magi, J. Am. Chem. Soc., 1986, 108, 1730–1735 CrossRef CAS.
- S. L. Lawton, A. S. Fung, G. J. Kennedy, L. B. Alemany, C. D. Chang, G. H. Hatzikos, D. N. Lissy, M. K. Rubin, H. K. C. Timken, S. Steuernagel and D. E. Woessner, J. Phys. Chem., 1996, 100, 3788–3798 CrossRef CAS.
- P. Mériaudeau, A. Tuel and T. T. H. Vu, Catal. Lett., 1999, 61, 89–92 CrossRef.
- G. L. Woolery, G. H. Kuehl, H. C. Timken, A. W. Chester and J. C. Vartuli, Zeolites, 1997, 19, 288–296 CrossRef CAS.
- H. Schulz, W. Böhringer, W. Baumgartner and Z. Siwei, Stud. Surf. Sci. Catal., 1986, 28, 915–922 CrossRef CAS.
- P. A. Russo, M. M. L. R. Carrott, P. J. M. Carrott, P. Matias, J. M. Lopes, M. Guisnet and F. R. Ribeiro, Microporous Mesoporous Mater., 2009, 118, 473–479 CrossRef CAS PubMed.
- H. Du, M. Kalyanaraman, M. A. Camblor and D. H. Olson, Microporous Mesoporous Mater., 2000, 40, 305–312 CrossRef CAS.
- X. Zhu, S. Liu, Y. Song, S. Xie and L. Xu, Appl. Catal., A, 2005, 290, 191–199 CrossRef CAS PubMed.
- M. Guisnet, P. Magnoux and D. Martin, Stud. Surf. Sci. Catal., 1997, 111, 1–19 CrossRef CAS.
- A. de Lucas, P. Canizares, A. Durán and A. Carrero, Appl. Catal., A, 1997, 156, 299–317 CrossRef CAS.
- P. Matias, J. M. Lopes, S. Laforge, P. Magnoux, P. A. Russo, M. M. L. Ribeiro Carrott, M. Guisnet and F. Ramôa Ribeiro, J. Catal., 2008, 259, 190–202 CrossRef CAS PubMed.
- A. Feller, J. O. Barth, A. Guzman, I. Zuazo and J. A. Lercher, J. Catal., 2003, 220, 192–206 CrossRef CAS.
- J. Ryczkowski, Catal. Today, 2001, 68, 263–381 CrossRef CAS.
- I. Kiricsi, H. Förster, G. Tasi and J. B. Nagy, Chem. Rev., 1999, 99, 2085–2114 CrossRef CAS PubMed.
- J. W. Park, J. Y. Lee, K. S. Kim, S. B. Hong and G. Seo, Appl. Catal., A, 2008, 339, 36–44 CrossRef CAS PubMed.
- Q. Qian, D. Mores, J. Kornatowski and B. M. Weckhuysen, Microporous Mesoporous Mater., 2011, 146, 28–35 CrossRef CAS PubMed.
- H. Forster, J. Seebode, P. Fejes and I. Kiricsi, J. Chem. Soc., Faraday Trans. 1, 1987, 83, 1109–1117 RSC.
- S. M. K. Airaksinen, M. A. Bañares and A. O. I. Krause, J. Catal., 2005, 230, 507–513 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00048c |
| This journal is © The Royal Society of Chemistry 2015 |