The key effect of the self-assembly mechanism of dendritic gelators: solubility parameters, generations and terminal effects†
Abstract
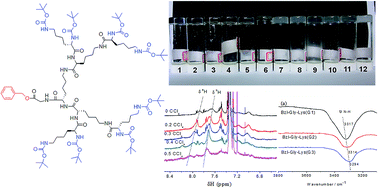
The key effect of the self-assembly mechanism of dendritic gelators is researched by a comprehensive investigation of the gelation behavior of L-lysine dendritic gelators with different structures of three generations in 20 different solvents. The solvents investigation, 1H NMR, tube inversion method, DSC, rheology, FTIR and rheological measurements show that the reported dendritic gelators self-assemble through the main driving force of hydrogen bonds and the second driving force of π–π stackings. So the key effect of the self-assembling mechanism is that these factors can influence the driving force of the self-assembly process. This is the reason that L-lysine dendritic gelators tend to gelate in solvents with low α and β parameter values, which have less influence on the formation of hydrogen bonds between the gelators. Higher generations provide a much greater hydrogen bond density in the gelators, which makes them have a higher gelation ability. The benzyl terminal groups provide the second driving force of π–π stacking, making the Bzl-Gly-Lys gelators have much stronger gelation ability. This research reports a comprehensive insight into the precise ways in which the solubility parameters of the solvents, the gelator generation and the terminal group effects can influence the self-assembly and gelation of dendritic gelators. Gaining this type of fundamental understanding is essential if the key effect of this important class of self-assembling soft materials is to be truly understood.

 Please wait while we load your content...
Please wait while we load your content...