Preparation of novel carbon nanofibers with BiOBr and AgBr decoration for the photocatalytic degradation of rhodamine B†
Guohua Jiang*abc,
Zhen Weia,
Hua Chena,
Xiangxiang Dua,
Lei Lia,
Yongkun Liua,
Qin Huanga and
Wenxing Chenabc
aDepartment of Materials Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, P. R. China. E-mail: ghjiang_cn@zstu.edu.cn; Tel: +86 571 86843527
bNational Engineering Laboratory for Textile Fiber Materials and Processing Technology (Zhejiang), Hangzhou 310018, P. R. China
cKey Laboratory of Advanced Textile Materials and Manufacturing Technology (ATMT), Ministry of Education, Hangzhou 310018, P. R. China
First published on 20th March 2015
Abstract
Novel carbon nanofibers with BiOBr and AgBr decoration have been prepared by a combination of electrospinning, carbonization and solvothermal treatments. BiOBr/AgBr hybrids interweaved together and covered the carbon nanofibers to form a three-dimensional (3D) open porous structure. The resultant composite carbon nanofibers exhibited a high efficiency for the photocatalytic degradation of RhB in aqueous solution and were convenient to separate from water.
During recent decades, photocatalysis has attracted much attention in environmental restoration as a green and sustainable technology.1 Compared with other photocatalysts, the semiconductor photocatalytic process has shown great potential applications due to its lack of toxicity, low cost, high photocatalytic activity and photostability.2 The ability of this advanced oxidation technology to remove persistent organic compounds and microorganisms in water has been widely demonstrated. Despite these advantages, the practical applications of semiconductor photocatalysts need to deal with three major disadvantages: (1) photocatalytic nanoparticles easily form aggregates to minimize their surface area because of their high surface energy, which is unfavorable for photocatalytic reactions; (2) it is very difficult to separate photocatalytic nanoparticles from treated water by conventional methods (including centrifugation and filtration), which may lead to a loss of the photocatalyst and bring about secondary pollution; (3) the conventional semiconductors (e.g. TiO2 and ZnO) are restricted by their deficient visible light absorption or high recombination rate of the photogenerated carriers. To solve these problems, an ideal way is to grow these photocatalytic nanoparticles with visible light responsivity on certain substrates in the form of an ordered film without agglomeration.3 Aiming at effectively utilizing visible light, a great deal of effort has also been devoted to hierarchical structure development and band gap regulation because the shape and band energy of photocatalysts have vital influences on their physical/chemical properties.4 Among these, bismuth oxyhalide compounds have attracted considerable attention due to their remarkable photocatalytic activities under visible-light illumination,5 and their optical and catalytic properties can be modified by the incorporation of other highly reactive components, such as cations, anions, metal oxides and metal nanoparticles.6
Herein, we report a facile preparation of novel carbon nanofibers decorated with BiOBr and AgBr. Carbon nanofibers are flexible, conductive, and stable in corrosive conditions, and they can supply a large surface area, which is critical for nanostructure-based photovoltaic technology.3c They also have good heat and fatigue resistance. Moreover, the synergistic effect of BiOBr, AgBr and carbon nanofibers will greatly retard the recombination of photoinduced electrons and holes, which could significantly enhance the photocatalytic performance of hybrid composite nanofibers. Fig. 1 shows a schematic representation of the growth process of a BiOBr/AgBr hybrid on carbon nanofibers (see detailed preparation in the ESI†). Firstly, a spinning solution containing polyacrylonitrile (PAN) and BiCl3 with N,N-dimethylformamide (DMF) as the solvent was developed to produce composite PAN nanofibers via electrospinning. After the heat treatment, the PAN nanofibers were carbonized to form carbon nanofibers and Bi2O3 and Bi nanoparticles were immobilized on the carbon nanofibers due to the oxidation of the BiCl3 and reduction of the PAN at high temperature (see Fig. S1 and S2 in the ESI†).7 The Bi2O3 and Bi nanoparticles immobilized on the carbon nanofibers can be utilized as seeds to grow BiOBr/AgBr hybrids that have a stronger photocatalytic ability than pure BiOBr.8 These BiOBr/AgBr hybrids were further assembled into hierarchical architectures on the surface of the carbon nanofibers by a solvothermal method.
 | ||
| Fig. 1 The schematic representation of the growth process of the BiOBr/AgBr hybrid nanosheets on carbon nanofibers. | ||
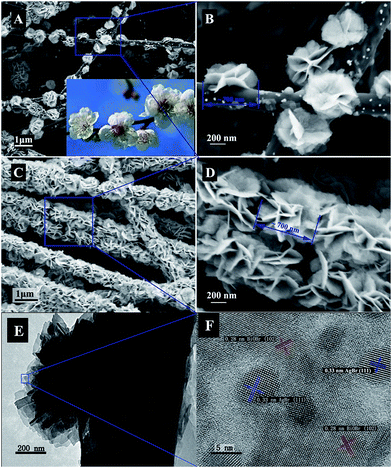
The morphology and structure of the carbon nanofibers with the BiOBr/Ag hybrid decoration were first characterized by field-emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM). The carbonized composite nanofibers were immersed in a solution of Bi(NO3)3, AgNO3 and CTAB. After the solvothermal treatment at 160 °C for 1 h, carbon nanofibers covered with the BiOBr/AgBr hybrids can be observed. As shown in Fig. 2A, these isolated BiOBr/AgBr hybrids with a flower-like structure are distributed on the surface of the carbon nanofibers. It looks like the plum blossoms on the heads of tree branches (inset in Fig. 2A). The size of the flower-like BiOBr/AgBr hybrids was around 700 nm. They consisted of spindle-like nanosheets of ∼200 nm in width and ∼5 nm in thickness (Fig. 2B). Prolonging the solvothermal treatment time to 6 h, the BiOBr/AgBr hybrids uniformly and compactly covered the nanofibers to form a rough surface (Fig. 2C and Fig. S3 in the ESI†). Through further magnification of the SEM image of the as-prepared composite nanofibers, it can be seen that these nanosheets interweaved together to form an open porous structure (Fig. 2D). It has been reported that such small sizes of these nanosheets may be indicative of a possible quantum confinement effect on the properties of such structures.9 TEM measurements were applied to analyze the composition and structure of the nanosheets. Fig. 2E shows a TEM image of the BiOBr/AgBr hybrid carbon nanofibers. The nanosheets are compactly grown on the surface of the carbon nanofibers. A selected area electron diffraction (SAED) pattern taken from the edge of the nanosheets is shown in Fig. 2F. It reveals the obvious lattice spacing of d = 0.28 nm, which is close to the d-spacing of the [102] (0.21 nm) reflections of pure BiOBr.10 The slight distinction in the lattice spacing is due to the presence of a dopant. Another lattice spacing of d = 0.33 nm is contributed from the [111] reflections of AgBr.11
The crystallographic structure of the composite nanofibers was further confirmed by powder X-ray diffraction (XRD) analysis. As shown in Fig. 3A, an obvious broad peak with 2θ from 10° to 20° can be observed from the carbon nanofibers, which is assigned to amorphous carbon. The diffraction peaks of the carbon nanofibers at 27.2°, 37.2°and 39.3° are in good agreement with the (012), (104) and (110) of the hexagonal Bi phase (JCPDS, 85-1329). Some weak and broad additional peaks also appeared, which match the crystal planes of Bi2O3 (JCDPS, 74-1375): (220), (013), (600) and (145) at 2θ = 24.5, 26.0, 53.9 and 56.2.12 This indicates that the BiCl3 has been reduced and oxidized simultaneously during the carbonization. After the solvothermal treatment, the diffraction peaks can be indexed to the tetragonal phase BiOBr (JCDPS, 09-0393, 2θ = 25.2, 32.2 46.2 and 57.1 corresponding well to (001), (110), (200) and (212).13 The XRD pattern of the BiOBr/AgBr hybrid carbon nanofibers also exhibits some weak dopant related peaks from AgBr (JCDPS, 06-0438) besides the typical tetragonal structure of the BiOBr crystal due to the low content and high dispersity of the dopant. No diffraction peaks of metal Ag are observed. The X-ray photoelectron spectrum of the photocatalyst exhibits prominent peaks of carbon, oxygen, bismuth, and bromine, and relatively feeble peaks of nitrogen and silver, as shown in Fig. 3B. The high-resolution XPS spectrum of Ag 3d from the composite photocatalyst is shown in Fig. 3C, which can be fitted as the two peaks at the binding energies of 367.2, and 373.2 eV, respectively, suggesting the presence of AgBr. As for the high resolution XPS spectrum of Br 3d that is shown in Fig. 3D, the binding energies of 67.8–68.3 eV and 68.7–69.2 eV can be observed. They are attributed to Br 3d5/2 and 3d3/2 respectively and can be assigned to Br in the monovalent oxidation state.14 The overlapped peak of Br 3d at the higher binding energy (∼68.9 eV) is due to the crystal lattice of Br in AgBr.15 These results are in agreement with the HR-TEM and XRD analysis.
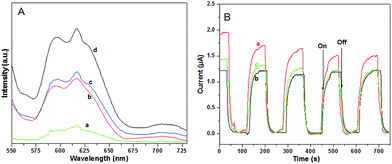
Photoluminescence (PL) analysis was used to reveal the efficiency of the charge carrier trapping, transfer, and separation, and to investigate the fate of the photogenerated electrons and holes in the composite carbon nanofibers, because the PL emission results from the recombination of free charge carriers.16 Herein, we present a suitable PL measurement for the carbon nanofibers covered with BiOBr or BiOBr/AgBr hybrids and the physical mixture of the carbon nanofibers and BiOBr/AgBr hybrids, as shown in Fig. 4A. A broad PL emission spectrum was observed for all products. However, in comparison with the carbon nanofibers covered with BiOBr (curve b), the intensity of the PL signal for the carbon nanofibers covered with BiOBr/AgBr hybrids is much lower (curve a). This indicates that the composite carbon nanofibers have a lower recombination rate of electrons and holes, due to the fact that the electrons are excited from the valence band to the conduction band and then transferred to the carbon nanofibers, preventing the direct recombination of electrons and holes. It also implies that electron–hole recombination on the surface of the composite carbon nanofibers is largely inhibited to generate more photoelectrons and holes to participate in the photocatalytic reaction.17 In the case of the physical mixture of the carbon nanofibers and BiOBr/AgBr hybrids, the intensity of the PL signal is close to the BiOBr/AgBr hybrids (curve c) and much higher than that of the composite (curve a). This suggests the carbon nanofibers are not accelerating the electron transfer due to no close connection between the carbon nanofibers and the BiOBr/AgBr hybrids. The photocurrent responses of the carbon nanofibers covered with BiOBr and BiOBr/AgBr hybrids under visible light (λ > 420 nm), are shown in Fig. 4B. The photocurrent intensity remains at a constant value when the light is on and rapidly decreases to zero as long as the light is turned off. It can clearly be observed that the photocurrent over the BiOBr/AgBr hybrid carbon nanofibers is greatly improved, which is about 1.3 times as high as that of the BiOBr/AgBr hybrids, because the photocurrent is formed mainly by the diffusion of photogenerated electrons to the back contact and simultaneously holes are taken up by the hole acceptor in the electrolyte.18 The enhanced photocurrent over the BiOBr/AgBr hybrid carbon nanofibers implies a more efficient separation of the photoinduced electron–hole pairs and a longer lifetime of the photogenerated charge carriers than that over the BiOBr/AgBr hybrids, which is beneficial for its enhanced photocatalytic activity. However, in the case of the physical mixture of the carbon nanofibers and BiOBr/AgBr hybrids, the photocurrent intensity is lower than the composite and close to that of BiOBr (curve c). This further confirms the higher separation efficiency of the photoinduced electron–hole pairs in the composite.
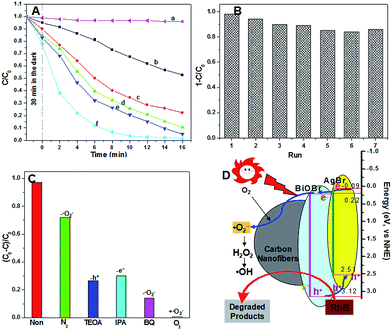
The photocatalytic activity of the as-prepared products was further evaluated by the degradation of rhodamine B (RhB, CRhB = 10 mg L−1) under visible light irradiation, as shown in Fig. 5A. For comparison, a blank experiment was first carried out to indicate the self-photodegradation of RhB. It was found that the self-photodegradation of RhB is almost negligible under light irradiation without any catalysts. Under light irradiation, the decolorization rate of RhB can be accelerated in the presence of carbon nanofibers, which can be ascribed to the dual actions of photolysis and adsorption.19 The decolorization rate of RhB is further accelerated in the presence of the BiOBr or BiOBr/AgBr composite photocatalysts. There is no doubt that here photocatalysis plays an important role to decolor RhB besides photolysis and adsorption. The fastest decolorization rate was obtained using the BiOBr/AgBr hybrid carbon nanofibers as the photocatalytic material. The color of the RhB solution became almost colorless within 10 min. Compared with the decolorization rate of the physical mixture of the carbon nanofibers and BiOBr/AgBr hybrids (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight), the carbon nanofibers play an important role to decolor RhB which improves the synergistic effects between photolysis and adsorption. The presence of the BiOBr/AgBr hybrids on the surface of the carbon nanofibers enlarges their specific surface area due to the nanosheets interweaving together to form an open porous structure, and thus, increasing the adsorption capacity. Nitrogen adsorption–desorption isotherm curves (see Fig. S4 in the ESI†) were carried out to further investigate the porous structure of the products. The BiOBr/AgBr hybrid carbon nanofibers have the highest specific surface area compared to the pure BiOBr and carbon nanofibers, owing to the pores produced by the association of the smaller nanosheets which has a positive role for the improvement of the photocatalytic activity. Meanwhile, the pre-enriched RhB molecules can be excited by light, and then the photoinduced electrons inject into the conduction band of the BiOBr/AgBr hybrids, triggering the photo-degradation reactions. The stability and reusability of catalysts are very important issues for practical applications. The activity of the BiOBr/AgBr hybrid carbon nanofibers was monitored for seven cycles under the same conditions for 20 min after simple separations and drying. As shown in Fig. 5B, no significant change in the photocatalytic activity was observed, indicating the durability of our separable photocatalyst in the degradation of RhB. It can be seen that the catalyst does not exhibit a significant loss of activity in seven successive runs. The degradation of RhB remains higher than 90% in each cycle, confirming the BiOBr/AgBr hybrid carbon nanofibers are not photocorroded and rather are stable during the photocatalytic reaction. The excellent reuse performance of the BiOBr/AgBr hybrid carbon nanofibers may result from the good binding property between the BiOBr/AgBr hybrid carbon nanofiber layer and the carbon nanofibers.
1 by weight), the carbon nanofibers play an important role to decolor RhB which improves the synergistic effects between photolysis and adsorption. The presence of the BiOBr/AgBr hybrids on the surface of the carbon nanofibers enlarges their specific surface area due to the nanosheets interweaving together to form an open porous structure, and thus, increasing the adsorption capacity. Nitrogen adsorption–desorption isotherm curves (see Fig. S4 in the ESI†) were carried out to further investigate the porous structure of the products. The BiOBr/AgBr hybrid carbon nanofibers have the highest specific surface area compared to the pure BiOBr and carbon nanofibers, owing to the pores produced by the association of the smaller nanosheets which has a positive role for the improvement of the photocatalytic activity. Meanwhile, the pre-enriched RhB molecules can be excited by light, and then the photoinduced electrons inject into the conduction band of the BiOBr/AgBr hybrids, triggering the photo-degradation reactions. The stability and reusability of catalysts are very important issues for practical applications. The activity of the BiOBr/AgBr hybrid carbon nanofibers was monitored for seven cycles under the same conditions for 20 min after simple separations and drying. As shown in Fig. 5B, no significant change in the photocatalytic activity was observed, indicating the durability of our separable photocatalyst in the degradation of RhB. It can be seen that the catalyst does not exhibit a significant loss of activity in seven successive runs. The degradation of RhB remains higher than 90% in each cycle, confirming the BiOBr/AgBr hybrid carbon nanofibers are not photocorroded and rather are stable during the photocatalytic reaction. The excellent reuse performance of the BiOBr/AgBr hybrid carbon nanofibers may result from the good binding property between the BiOBr/AgBr hybrid carbon nanofiber layer and the carbon nanofibers.
To investigate a plausible reaction mechanism for the superior photocatalytic activity of the BiOBr/AgBr hybrid carbon nanofibers and detection of the active species during the photocatalytic reactivity, hydroxyl radicals (˙OH), superoxide radical (˙O2−), and holes (h+) were investigated by adding 1.0 mM isopropyl alcohol (IPA, a quencher of ˙OH), p-benzoquinone (BQ, a quencher of ˙O2−), and triethanolamine (TEOA, a quencher of h+), respectively (see details in the ESI†).20 It was found that with the addition of 1 mM BQ into the reaction system, the decolorization rate of RhB was decelerated significantly compared with the addition of 1.0 mM of IPA or TEOA. Therefore, it can be concluded that ˙O2− plays an important role in the degradation of the organic pollutant solution under light irradiation, as shown in Fig. 5C. When N2 was bubbled into the reaction system, the degradation of RhB over the BiOBr/AgBr hybrid composite carbon nanofibers was decelerated. However, complete degradation of RhB occurred under the same conditions but bubbled with O2. These phenomena reveal that molecular oxygen has an important effect on the photocatalytic degradation of RhB over the BiOBr/AgBr hybrid composite carbon nanofibers.21 It indicates that ˙O2− is an even more efficient oxidizer, which results in the oxidation and eventual mineralization of organic compounds.22
From the analysis above, it can be concluded that light irradiation activates the BiOBr/AgBr hybrids to generate strongly oxidative holes (h+) in the valence band and reductive electrons (e−) in the conduction band. Then, these photoinduced electrons are trapped by dissolved oxygen (O2) to yield superoxide ions (˙O2−) and H2O2 and then hydroxyl radicals (˙OH).21 On the other hand, electron transfer between the BiOBr/AgBr hybrids and the carbon nanofibers will greatly retard the recombination of photoinduced charge carriers and prolong the electron lifetime, which may be an important role for the excellent photoactivity of the products.9a,23 Based on the above results, it can be concluded that photogenerated holes and ˙OH are the major species active for the photodegradation of RhB, and ˙O2− is just an intermediate to produce ˙OH but is not involved directly in the degradation of RhB. This explains the phenomena that the effect of bubbling N2 and O2 into the reacting solution on the photodegradation of RhB is markedly inhibited and enhanced, respectively (Fig. 5D). The roles of the carbon nanofibers in the composite during the photocatalytic reaction can be summarized as follows: (1) as a substrate for the immobilization of the BiOBr/AgBr hybrids; (2) as a conductor for the acceleration of the electron transfer; (3) enhancement of the synergistic effect between photolysis and adsorption.
Conclusions
In summary, carbon nanofibers have been prepared by the carbonization of PAN nanofibers. BiOBr/AgBr hybrids with a flower-like nanosheet structure were further immobilized on the surface of the carbon nanofibers by a facile solvothermal route. The morphologies, structural properties, and photocatalytic activities of the resultant products were investigated. At the same time, it was also proved that the removal of organic pollutants from solutions was caused by photocatalytic degradation rather than by sorption. Furthermore, the three-dimensional (3D) BiOBr/AgBr hybrid structure can capture light from all directions, thus showing potential for applications in places with a high albedo (high fraction of reflected radiation). This work may provide new insights into preparing other inorganic photocatalytic fibers and may extend their potential applications for the degradation of organic pollutants.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51373155, 51133006), Public Technology Research Project of Zhejiang Province (2014C33G2060070) and “521 Talents Training Plan” in Zhejiang Sci-Tech University (ZSTU).Notes and references
- Z. Liu, Y.-E. Miao, M. Liu, Q. Ding, W. W. Tjiu, X. Cui and T. Liu, J. Colloid Interface Sci., 2014, 424, 49–55 CrossRef CAS PubMed.
- (a) I. K. Konstantinou and T. A. Albanis, Appl. Catal., B, 2004, 49, 1–14 CrossRef CAS PubMed; (b) G. Jiang, X. Li, Z. Wei, T. Jiang, X. Du and W. Chen, Powder Technol., 2014, 260, 84–89 CrossRef CAS PubMed; (c) R. Wang, G. Jiang, Y. Ding, Y. Wang, X. Sun, X. Wang and W. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 4154–4158 CrossRef CAS PubMed.
- (a) H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed; (b) G. Jiang, X. Wang, Z. Wei, X. Li, X. Xi, R. Hu, B. Tang, R. Wang, S. Wang, T. Wang and W. Chen, J. Mater. Chem. A, 2013, 1, 2406–2410 RSC; (c) W. Guo, F. Zhang, C. Lin and Z. L. Wang, Adv. Mater., 2012, 24, 4761–4764 CrossRef CAS PubMed.
- R. Wang, G. Jiang, X. Wang, R. Hu, X. Xi, S. Bao, Y. Zhou, T. Tong, S. Wang, T. Wang and W. Chen, Powder Technol., 2012, 228, 258–263 CrossRef CAS PubMed.
- (a) Z. Ai, W. Ho, S. Lee and L. Zhang, Environ. Sci. Technol., 2009, 43, 4143–4150 CrossRef CAS; (b) M. A. Gondal, X. Chang, M. A. Ali, A. H. Yamani, Q. Zhou and G. Ji, Appl. Catal., A, 2011, 397, 192–200 CrossRef CAS PubMed; (c) H. Cheng, B. Huang, P. Wang, Z. Wang, Z. Lou, J. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054–7056 RSC; (d) Y. Fang, Y. Huang, J. Yang, P. Wang and G. Cheng, Environ. Sci. Technol., 2011, 45, 1593–1600 CrossRef CAS PubMed; (e) T. Li, G. Chen, C. Zhou, Z. Shen, R. Jin and J. Sun, Dalton Trans., 2011, 40, 6751–6758 RSC; (f) W. Wang, F. Huang, X. Lin and J. Yang, Catal. Commun., 2008, 9, 8–12 CrossRef CAS PubMed; (g) Y. Feng, L. Li, J. Li, J. Wang and L. Liu, J. Hazard. Mater., 2011, 192, 538–544 CrossRef CAS PubMed.
- (a) Z. Liu, H. Ran, J. Niu, P. Feng and Y. Zhu, J. Colloid Interface Sci., 2014, 431, 187–193 CrossRef CAS PubMed; (b) J. Li, Y. Yu and L. Zhang, Nanoscale, 2014, 6, 8473–8488 RSC; (c) C. Yu, F. Cao, G. Li, R. Wei, J. C. Yu, R. Jin, Q. Fan and C. Wang, Sep. Purif. Technol., 2013, 120, 110–122 CrossRef CAS PubMed.
- B. F. Dal, S. G. Hardin, D. G. Hay and T. W. Turney, J. Mater. Sci., 1993, 28, 6657–6664 CrossRef CAS.
- G. Jiang, R. Wang, X. Wang, R. Hu, X. Xi, Y. Zhou, S. Wang, T. Wang and W. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4440–4444 CAS.
- (a) G. Jiang, X. Li, Z. Wei, T. Jiang, X. Du and W. Chen, Powder Technol., 2014, 260, 84–89 CrossRef CAS PubMed; (b) G. Jiang, X. Li, Z. Wei, X. Wang, T. Jiang, X. Du and W. Chen, Powder Technol., 2014, 261, 170–175 CrossRef CAS PubMed; (c) J. Zhang, F. Shi, J. Lin, D. Chen, J. Gao, Z. Huang, X. Ding and C. Tang, Chem. Mater., 2008, 20, 2937–2941 CrossRef CAS; (d) G. Jiang, B. Tang, H. Chen, Y. Liu, L. Li, Q. Huang and W. Chen, RSC Adv., 2015, 5, 25801–25805 RSC.
- (a) C. Yu, C. Fan, X. Meng, K. Yang, F. Cao and X. Li, React. Kinet., Mech. Catal., 2011, 103, 141–151 CrossRef CAS PubMed; (b) L. Lu, L. Kong, Z. Jiang, L. Lu, L. Kong, Z. Jiang, H. H.-C. Lai, T. Xiao and P. P. Edwards, Catal. Lett., 2012, 142, 771–778 CrossRef CAS.
- H. Wang, J. Gao, T. Guo, R. Wang, L. Guo, Y. Liu and J. Li, Chem. Commun., 2012, 48, 275–277 RSC.
- Y. Zhao, Z. Zhang and H. Dang, Mater. Lett., 2004, 58, 790–793 CrossRef CAS PubMed.
- Z. Jiang, F. Yang, G. Yang, L. Kong, M. O. Jones, T. Xiao and P. P. Edwards, J. Photochem. Photobiol., A, 2010, 212, 8–13 CrossRef CAS PubMed.
- (a) K.-L. Li, W. W. Lee, C.-S. Lu, Y.-M. Dai, S.-Y. Chou, H.-L. Chen, H.-P. Lin and C.-C. Chen, J. Taiwan Inst. Chem. Eng., 2014, 45, 2688–2697 CrossRef CAS PubMed; (b) S. Wang, W. Ma, Y. Fang, M. Jia and Y. Huang, Appl. Catal., B, 2014, 150–151, 380–388 CrossRef CAS PubMed.
- L. Zhang, K.-H. Wong, Z. Chen, J. C. Yu, J. Zhao, C. Hu, C.-Y. Chan and P.-K. Wong, Appl. Catal., A, 2009, 363, 221–229 CrossRef CAS PubMed.
- X. Bai, L. Wang, Y. Wang, W. Yao and Y. Zhu, Appl. Catal., B, 2014, 152–153, 262–270 CrossRef CAS PubMed.
- L. Kong, Z. Jiang, T. Xiao, L. Lu, M. O. Jones and P. P. Edwards, Chem. Commun., 2011, 47, 5512–5514 RSC.
- X. Tu, S. Luo, G. Chen and J. Li, Chem.–Eur. J., 2012, 18, 14359–14366 CrossRef CAS PubMed.
- J. Shi, H. Cui, J. Chen, M. Fu, B. Xu, H. Luo and Z. Ye, J. Colloid Interface Sci., 2012, 388, 201–208 CrossRef CAS PubMed.
- (a) L. Ye, J. Liu and C. Gong, ACS Catal., 2012, 2, 1677–1683 CrossRef CAS; (b) L. Chen, S. Yin and S. Luo, Ind. Eng. Chem. Res., 2012, 51, 6760–6768 CrossRef CAS.
- J. Ma, L.-Z. Zhang, Y.-H. Wang, S.-L. Lei, X.-B. Luo, S.-H. Chen, G.-S. Zeng, J.-P. Zou, S.-L. Luo and C.-T. Au, Chem. Eng. J., 2014, 251, 371–380 CrossRef CAS PubMed.
- (a) J. Xiao, Y. Xie and H. Cao, Chemosphere, 2015, 121, 1–17 CrossRef CAS PubMed; (b) Y. Li, J. Niu, L. Yin, W. Wang, Y. Bao, J. Chen and Y. Duan, J. Environ. Sci., 2011, 23, 1911–1918 CrossRef CAS.
- W. Cui, S. Ma, L. Liu, J. Hu, Y. Liang and J. G. McEvoy, Appl. Surf. Sci., 2013, 271, 171–181 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of materials, preparation of composite carbon nanofibers, preparation of BiOBr/AgBr composite carbon nanofibers, characterization, measurement of photocatalytic activity, SEM images of PAN/BiCl3 composite nanofibers before and after carbonization, SEM images of BiOBr/AgBr hybrid composite carbon nanofibers obtained at different reaction times, N2 adsorption–desorption isotherm curves. See DOI: 10.1039/c4ra17290f |
| This journal is © The Royal Society of Chemistry 2015 |