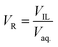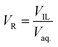Treatment of energetic material contaminated wastewater using ionic liquids†
Vikas K. Bhosale,
Nitin V. Patil and
Prashant S. Kulkarni*
Energy and Environment Laboratory, Department of Applied Chemistry, Defence Institute of Advanced Technology, Deemed University, Girinagar, Pune-411025, India. E-mail: ps_kulkarni@rediffmail.com; pskulkarni@diat.ac.in; Fax: +91 20 24389509; Tel: +91 20 24304161
First published on 13th February 2015
Abstract
The contamination of energetic materials (EMs) in wastewater during its production and application is one of the serious environmental issues. Selected hydrophobic ionic liquids (ILs) were developed, characterized and applied for the separation of EMs such as TNT, tetryl and picric acid from aqueous medium. Batch-wise extraction experiments were performed at 700 rpm in a capped glass vial at 25 °C. The IL containing anion, bis(trifluoromethanesulfonyl)imide ([NTf2]−) played a vital role in the separation of EMs due to its large ionic radius. It was, therefore, extensively used for the optimization of various process parameters. The investigation of the extraction mechanism has revealed that a C–H⋯Π interaction is present between the IL and EM. The results show the highest distribution ratio of 2095 for the tetryl amongst the other EMs used. A maximum separation factor for tetryl/TNT, TNT/picric acid and picric acid/tetryl was observed to be 1.75, 6.65 and 0.15, respectively for the IL, 1-octyl-3-methylimidazolium bis[trifluoromethanesulfonyl] imide ([OMIM][NTf2]). After the extraction, the IL was separated from the aqueous phase and repeatedly treated five times with a fresh batch of EM.
1. Introduction
Every year enormous quantities of energetic materials (EMs) such as TNT (2,4,6 trinitrotoluene), RDX (hexahydro-1,3,5-trinitro-1,3,5 triazine) and tetryl (2,4,6-trinitro-phenylmethylnitramine) are being developed and widely used for several strategic applications.1,2 These include rocket and gun propellants, missiles, high explosives and pyrotechnics.3 Some of them are also found to be useful in various industrial activities viz. mining, underwater blasting, dyestuff, precursor in synthetic leather, agricultural and intermediate in the manufacture of photographic materials.4 Their large-scale production and varied usage contaminate the water and soil.5–7 The EMs are mobile in ground water and soil and hence, it pollutes the environment system.8 They mainly contain nitroaromatics and nitramines which are very toxic and produce mutagenic effects on a living organism including humans and animals. This causes liver damage, gastritis and aplastic anemia, cyanosis, dermatitis and inhibits the growth of fungi, yeast, actinomyces and Gram-positive bacteria.9 The degraded products of EMs also show an adverse effect on the aquatic environment. It is, therefore, necessary to treat EMs bearing wastewater prior to release in the environment.The USEPA has ambient criteria of 0.06 mg L−1 for TNT and 0.3 mg L−1 for RDX in aqueous stream and in drinking water criteria of 0.049 mg L−1 for TNT, 0.02 mg L−1 for tetryl and 0.035 mg L−1 for RDX. Recently, USEPA has proposed a lifetime health advisory level of 0.002 mg L−1 for TNT in drinking water.10 Therefore, concentration of total nitro-organics in the effluent wastewater needs to be brought down, so as to meet the stringent discharge limits. Current processes for the treatment of EMs bearing aqueous streams involve adsorption,11,12 biodegradation,13,14 degradation by advanced oxidation process,6,15 supercritical water,16,17 precipitation,18 vacuum distillation,19 and molecular imprinted polymer.20 Solvent extraction is one of such process, which is easy, simple and quiet economical.21 Nevertheless, the conventional organic solvents used are volatile, toxic and flammable. It is desirable and worthwhile to explore new solvents for the handling of high EMs-laden aqueous stream.
In recent years, ionic liquids (ILs) are claimed to be potential solvents to replace conventional organic solvents in liquid–liquid extraction process.22,23 ILs are salts having a melting point below 100 °C. They are stable in the presence of air and water and have low vapor pressure, large liquid range, good thermal and chemical stability.24 It is, therefore, ILs have shown considerable promise as a new alternative solvent for extract and to separate various metal ions25–28 and organic compounds29–33 from aqueous streams. They were also used for the removal of refractory sulfur compounds from diesel fuel.34–37 In the present work, we report on the extraction of EMs from aqueous streams by using ILs.38 The principal objective of this investigation was to examine the feasibility of ILs for the treatment of EMs contaminated water. For this purpose, the influence of various operating parameters on the extracting behavior of IL, including extraction kinetics, effect of the cation and anion, pH of the aqueous stream and phase volume ratios were evaluated.
2. Materials and methods
2.1. Chemicals
The chemicals required for the preparation ILs include 1-methyl imidazole (99%, Aldrich), 1-chlorooctane (99%, Aldrich), 1-butyl 3-methyl imidazolium chloride (99%, Sigma-Aldrich), diethyl ether (puriss. pa., ACS reagent ≥99.8%, Sigma-Aldrich), potassium hexafluorophosphate (98%, Sigma-Aldrich), lithium bis(trifluoromethanesulfonyl)imide (Merck Schuhardt OHG), magnesium sulphate (anhydrous, reagent grade, ≥97%, Sigma-Aldrich), ethyl acetate (≥99.5%, Merck) and dichloromethane (anhydrous ≥ 99.8%, Sigma-Aldrich). All the EMs were obtained from Ordnance Factory Khadaki, Pune. Deionised water (TKA smart 2 pure, Thermofisher Scientific) was used for the purification of ILs, preparation of EMs stock solution and solvent for HPLC. The pH (HANNA HI 2211, pH Meter) of the aqueous solution was adjusted by using 1 M H2SO4 (95–97%, Fluka) and 1 M NaOH (97.0%, Fisher Scientific). HPLC grade ≥99.9% acetonitrile was obtained from Sigma-Aldrich. All the chemicals were used as received, without further purification.2.2. Preparation and characterization of ionic liquids
The ILs, 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]), 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([BMIM][NTf2]), 1-octyl-3-methylimidazolium chloride ([OMIM][Cl]), 1-octyl-3-methylimidazolium hexafluorophosphate ([OMIM][PF6]) and 1-octyl-3-methylimidazolium bis[trifluoromethanesulfonyl]imide ([OMIM][NTf2]) were synthesized according to the method described elsewhere.39 Their purification was done by the dissolution of the organic layer into the solvent, dichloromethane. This phase was washed with distilled water, dried over magnesium sulphate and filtered. The solvent was removed by rotary vacuum evaporator and dried under high vacuum at 70 °C for 24 h. The purification of ILs was confirmed by using various analytical tools. For instance, the water content of ILs was measured by Karl-Fisher Autotitrator (LABINDIA TITRA, TP13020201) and was found to be less than 0.01%. Their density was measured by the Anton-Parr density meter (DMA 500). Further, the ILs were characterized by FT-IR (BRUKAR, ALPHA FT-IR spectrometer with Zn–Se ATR crystal and gold coated mirror) and the 1H and 13C NMR spectra were recorded on a Varian Mercury 300 MHz and 75 MHz spectrometer with tetramethylsilane as an internal standard and DMSO-d6 as a solvent. The solubility of ILs was tested in common solvents. The physico-chemical properties of ILs39–41 are given in Table 1S† and the spectra, and data analysis are given in the ESI.2.3. Extraction of energetic materials
For the extraction purpose, TNT, tetryl and picric acid were selected as model EMs. The aqueous stream was prepared by dissolving the EM into the deionised water. The ILs, [BMIM][PF6], [BMIM][NTf2], [OMIM][PF6] and [OMIM][NTf2] were selected for the liquid–liquid extraction of EMs. A known quantity of predetermined EM concentration was taken for the extraction experiment. The phase volume ratio of IL (VIL) and aqueous phase (Vaq.) was adjusted according to the extraction parameter. The extraction was carried at 700 rpm in a capped glass vial at 25 °C. The pH and concentration of EMs were changed according to the experimental parameter. Aqueous sample of EM was analyzed by HPLC, before and after the extraction experiment. All the experiments were performed in duplicate.2.4. Analysis of energetic materials
The EMs containing TNT, tetryl and picric acid were analyzed as per the procedure described (USEPA method 8330)42 by using HPLC (model Hitachi). The chromatographic reverse phase C18 column was used with a size of 25 cm × 4.6 mm, 5 μm at 25 °C. A UV-Vis detector was used at a wavelength of 248 nm. The analytes were eluted isocratically at a flow-rate of 0.5 mL min−1, using an acetonitrile/water mobile phase (80/20, v/v). The sample injection volume used for the analysis was 20 μL. The retention time (Rt) of TNT, tetryl and picric acid were obtained at 7.1, 6.6 and 3.2 min, respectively. The calibration of TNT, tetryl and picric acid gave linear regression curve, which was obtained by plotting amplitude (Y, mAU) against the concentration (X, ppm). The curve exhibited good linearity, and the data graph is given in ESI. All aqueous samples of EMs were analyzed by this method.2.5. Distribution ratio (DX), percentage of extraction (%E) and separation factor (α) of energetic materials
The distribution ratio (DX) of EM ‘X’ in IL was calculated as
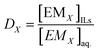 | (1) |
The concentration of EMs is denoted by [EMX], which is strongly extracted into the IL phase from the aqueous phase, the remaining concentration of EMs was measured after extraction; the eqn (1) can be rewritten as
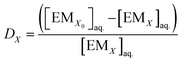 | (2) |
The volume of IL phase was denoted as VIL, and the volume of EMs in the aqueous phase was denoted as Vaq.. The EM was strongly extracted into the IL phase. After the extraction, concentration of EM in IL phase is ([EMX]IL) and the remaining concentration of EM in the aqueous phase is ([EMX]aq.). The fraction of EM (q) present in the aqueous phase after extraction was measured by following equation
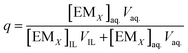 | (3) |
If definite volume ratio,
The eqn (3) is simplified as
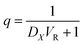 | (4) |
The amount of EM fraction (p) extracted by the IL phase is determined as
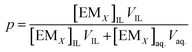 | (5) |
If definite volume ratio,
The eqn (5) simplified as
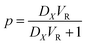 | (6) |
The percentage of extraction (%E) is defined as the amount of solute extracted to the organic phase over the total amount of solute in both phases.
The percentage of EM extracted by IL from aqueous phase is measured by following equation:
| %E = 100 × p | (7) |
Also, we can measure the percentage of EM remaining in the aqueous phase by following equation:
| %E = 100 × q | (8) |
In a mixture of EMs, the efficiency of separation between two EMs can be described with separation factor α
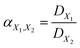 | (9) |
After the extraction, EM was striped from the ionic liquid by using strippant. The percentage recovery (%R) of EM in the stripping phase can be defined as amount of EM striped from the IL phase to the total amount of EM in the IL before stripping:
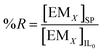 | (10) |
3. Results and discussion
3.1. Extraction kinetics
The extraction equilibrium time of EM in IL was measured by stirring 100 ppm of the aqueous stream of TNT with IL, [BMIM][NTf2]. The pH of the aqueous phase of TNT was found to be 6.3. The extraction experiment was conducted up to 60 minute with a fixed volume ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (VIL/Vaq.) in a capped glass vial for 700 rpm at 25 °C. After the completion of the experiment, the aqueous phase was carefully separated from the IL phase. Later, the sample was analyzed by HPLC, and the results were calculated by using the calibrated graphs. Fig. 1 demonstrates that more than 80% of extraction was achieved within 5 minutes, and a complete extraction was observed within 30 minutes. At these conditions, the distribution ratio of TNT for IL, [BMIM][NTf2] was observed to be 125.5.
3 (VIL/Vaq.) in a capped glass vial for 700 rpm at 25 °C. After the completion of the experiment, the aqueous phase was carefully separated from the IL phase. Later, the sample was analyzed by HPLC, and the results were calculated by using the calibrated graphs. Fig. 1 demonstrates that more than 80% of extraction was achieved within 5 minutes, and a complete extraction was observed within 30 minutes. At these conditions, the distribution ratio of TNT for IL, [BMIM][NTf2] was observed to be 125.5.
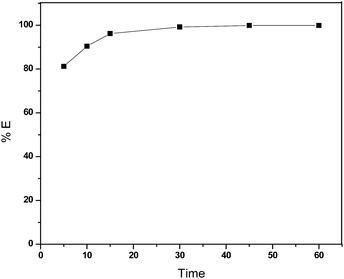 | ||
Fig. 1 Extraction of EM, TNT from aqueous stream using IL, [BMIM][NTf2] as a function of time under the condition of conc. of TNT 100 ppm, pH 6.3 and VIL/Vaq. 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3. | ||
3.2. Interaction of EM with ionic liquid
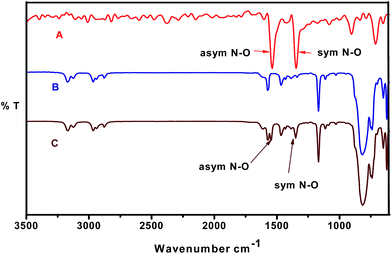
In order to get more information about the interaction of IL with the EM, the extracting samples were further analyzed by using FT-IR and NMR spectrometer. As shown in Fig. 2, the 1H NMR spectroscopic study gives strong evidence for the interaction of TNT and IL, [BMIM][NTf2]. The more electronegative C2-Ha of N-alkyl imidazolium cation and aromatic Π moiety of TNT can form the polar interactions. The interaction between the IL and EM is preferential via C–H⋯Π interaction and is confirmed by the NMR chemical shift of C2-Ha of N-alkyl imidazolium cation. The C2-Ha of N-alkyl imidazolium cation exhibited the greatest shielding (up to −0.45 ppm) indicating the existence of C2Ha–Π interaction of cation with EM, TNT.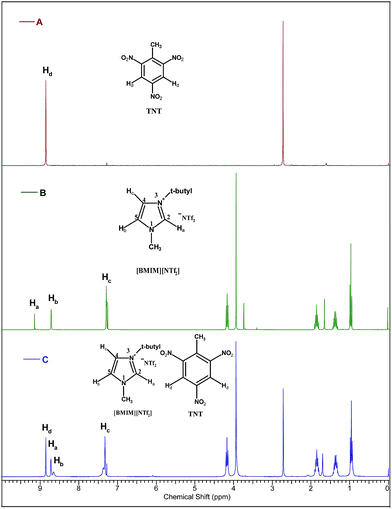 | ||
| Fig. 2 1H NMR spectra of (A) TNT (solvent CDCl3), (B) [BMIM][NTf2] (solvent DMSO-d6) and (C) TNT with [BMIM][NTf2] (solvent DMSO-d6). | ||
Further in a typical experiment, the interaction of nitro-group of TNT and IL, [BMIM][PF6] was investigated by using FT-IR and the results are shown in Fig. 3. The asymmetric stretching of N–O of TNT changes from 1537 to 1545 cm−1 and symmetric stretching of N–O of TNT changes from 1345 to 1349 cm−1. It specifies that decrease in the delocalization of nitro-group of TNT aromatic ring and increase in the interaction of Π-bond of the aromatic ring with the C2-Ha bond of IL cation. Therefore, both the analysis confirms the C–H⋯Π interaction between the IL and EM.
3.3. Types of ionic liquid
The ILs are made up of organic cation and organic or inorganic anion and hence, both of them can influence the separation of EMs. Therefore, it was thought desirable to study their effect on the extraction efficiency of EMs. The ILs, [BMIM][PF6], [BMIM][NTf2], [OMIM][PF6] and [OMIM][NTf2] were used for the extraction of TNT from the aqueous stream. The experiments were performed in a 10 mL glass vial with phase volume ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (VIL/Vaq.) and extraction time of 60 min. During the experiments, except the IL all other parameters were kept constant. The results as shown in Fig. 4 indicates that IL, [OMIM][NTf2] gives highest distribution ratio as compared to others.
3 (VIL/Vaq.) and extraction time of 60 min. During the experiments, except the IL all other parameters were kept constant. The results as shown in Fig. 4 indicates that IL, [OMIM][NTf2] gives highest distribution ratio as compared to others.
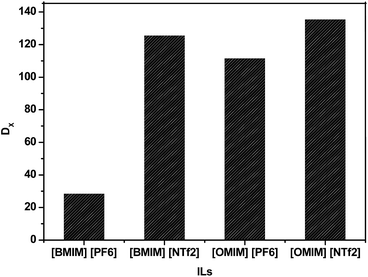 | ||
Fig. 4 Effect of types of IL on the distribution ratio of TNT under the condition of pH 6.3, VIL/Vaq. 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, conc. of TNT 100 ppm and extraction time 60 min. 3, conc. of TNT 100 ppm and extraction time 60 min. | ||
The demonstration further clarifies that distribution ratio of TNT was improved as the N-alkyl side chain length of imidazolium cation increases from C4 to C8 and with the same anion of ILs (i.e. [OMIM][NTf2] > [BMIM][NTf2] and [OMIM][PF6] > [BMIM][PF6]). This may be due to the increase in the N-alkyl side-chain length of the imidazolium cation increases the volume size of the channels formed by interaction of the imidazolium cations with anions.43 It further increases the possibility of polar interaction of TNT with N-alkyl chain of the imidazolium cation. Thus, improves the accommodation of more TNT molecules within IL, and in turn, increases the distribution ratio of TNT.
In the extraction process not only cation plays an important role but also the anion shows a significant contribution. It is observed that with the same cation the effect of anion varies extensively from [NTf2]− to [PF6]−. Amongst the selected ILs, it is found that the significant effects of anion on extraction efficiency of TNT was according to the order: [NTf2]− > [PF6]−. The anion [NTf2]− has a larger anionic radius than [PF6]−, and hence, the interaction strength between the cation and the anion decreases with the increase in anionic radius. This induces loose association between the imidazolium cation and anion [NTf2]−. Thus, anion [NTf2]− can provide stronger hydrogen bonding between IL and TNT which resulted in higher distribution ratio in case of [OMIM][NTf2] and [BMIM][NTf2].44 Another noted reason for decreasing the distribution ratio of anion [PF6]− is due to its instability upon contacting with water for longer duration.45,46
3.4. pH of the aqueous stream
The pH of the EMs bearing industrial wastewater varies widely. For studying the effect of pH, the extraction of TNT from the aqueous stream was investigated at pH 2 to 8 by using IL, [BMIM][NTf2]. A phase volume ratio (VIL/Vaq.) of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was used for liquid–liquid extraction experiment under the identical conditions of stirring speed and temperature. The pH of aqueous stream was maintained by adding H2SO4 and NaOH solutions. Aqueous stream of 100 ppm of TNT was contacted with the IL. It was observed that with decreasing the pH from its original value of 6.3 to 2, the distribution ratio of TNT increased from 56 to 96 which is as shown in Fig. 5. It may be attributed to a decrease in solubility of TNT as the pH value of aqueous stream decreases.47 A similar effect was observed during the extraction of TNT using toluene.48 The effect of pH was further investigated from 6.3 to 8, and it was observed that the distribution ratio of TNT was slightly decreased from 56 to 52. It could be due to the addition of hydroxide ions, which may have increased the solubility of TNT in water and, therefore, affected the distribution ratio of TNT in IL phase. From these investigations, it is clear that the extraction of TNT is enhanced by decreasing the pH value.
3 was used for liquid–liquid extraction experiment under the identical conditions of stirring speed and temperature. The pH of aqueous stream was maintained by adding H2SO4 and NaOH solutions. Aqueous stream of 100 ppm of TNT was contacted with the IL. It was observed that with decreasing the pH from its original value of 6.3 to 2, the distribution ratio of TNT increased from 56 to 96 which is as shown in Fig. 5. It may be attributed to a decrease in solubility of TNT as the pH value of aqueous stream decreases.47 A similar effect was observed during the extraction of TNT using toluene.48 The effect of pH was further investigated from 6.3 to 8, and it was observed that the distribution ratio of TNT was slightly decreased from 56 to 52. It could be due to the addition of hydroxide ions, which may have increased the solubility of TNT in water and, therefore, affected the distribution ratio of TNT in IL phase. From these investigations, it is clear that the extraction of TNT is enhanced by decreasing the pH value.
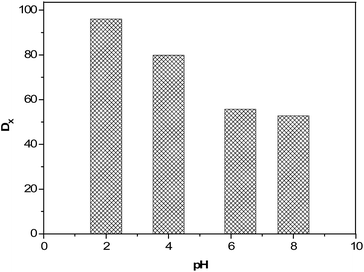 | ||
Fig. 5 Effect of pH on the distribution ratios of TNT in IL, [BMIM][NTf2] under the condition of conc. of TNT 100 ppm, VIL/Vaq. 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and extraction time 60 min. 3 and extraction time 60 min. | ||
3.5. Types of energetic material
In this study, we have compared the extraction performances of TNT, tetryl and picric acid with ILs, [BMIM][NTf2], [OMIM][NTf2], [OMIM][PF6] and [BMIM][PF6]. For this purpose, 50 ppm aqueous solutions of each EMs were separately contacted with the respective IL under the identical conditions. It is important to note that the neat pH of each aqueous solutions of TNT, tetryl and picric acid varies as 6.3, 7.5 and 4.5, respectively. All the ILs have shown considerable extraction capacities for the selected EMs as they undergo strong polar interaction or via the formation of inclusion type compounds with the aromatic EM moiety and facilitates the EM removal from the aqueous stream.The selectivity data reveals that the distribution ratio of tetryl is high over TNT and picric acid as shown in Table 1. It is because tetryl has additional nonbonding pair of electron on nitrogen. The nonbonding electron pair of tetryl enhances the interaction with the IL.43 On the contrary, the picric acid showed low distribution ratio which could be due to its high solubility in water as compared to the other EMs. This factor leads to the increase in hydration energy of picric acid and might have lowered the distribution of the same in IL phase. Therefore, it showed lowest distribution ratio as compared to the TNT and tetryl. Further, in a typical experiment, the selectivity of TNT and tetryl was calculated by using a mixture of both the EMs and the separation factor (α) of tetryl was found to be 1.65 over TNT. These results demonstrate that the IL also shows selectivity for the removal of EMs.
| Ionic liquids | Selectivitya | ||
|---|---|---|---|
| Tetryl/TNT | TNT/picric acid | Picric acid/tetryl | |
| a Determined on the basis of individual distribution ratios.b Observed selectivity based on extraction from an aqueous stream containing a mixture of tetryl and TNT. | |||
| [BMIM][PF6] | 1.32 | 5.85 | 0.12 |
| [BMIM][NTf2] | 1.62 (1.79)b | 6.11 | 0.14 |
| [OMIM][PF6] | 1.38 | 5.45 | 0.12 |
| [OMIM][NTf2] | 1.75 (1.84)b | 6.65 | 0.15 |
3.6. Phase volume ratio
The alteration of IL quantity would be an important operating variable. Consequently, the volume ratios (VIL/Vaq.) of IL to the aqueous phase were varied from 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. For this purpose, the IL, [BMIM][NTf2] was selected, and the experiments were performed under identical conditions. Fig. 6 demonstrates the influence of volume ratio of IL versus aqueous stream on the distribution ratio of TNT, tetryl and picric acid. It was observed that the distribution ratio of EMs gradually increases with an increase in the volume of IL. The highest distribution ratio was obtained at a phase volume ratio of 3
3. For this purpose, the IL, [BMIM][NTf2] was selected, and the experiments were performed under identical conditions. Fig. 6 demonstrates the influence of volume ratio of IL versus aqueous stream on the distribution ratio of TNT, tetryl and picric acid. It was observed that the distribution ratio of EMs gradually increases with an increase in the volume of IL. The highest distribution ratio was obtained at a phase volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. It is because the increase in phase volume ratio increases the number of moles of ILs and enhances the interaction with TNT, tetryl and picric acid and results in higher distribution ratio. Apparently, there exists an increasing trend of distribution ratios of all the EMs with increasing volume ratios of IL versus aqueous stream. The distribution ratio of EM from aqueous stream at high volume ratios was significantly superior to that low one i.e. 1234 versus 20 for TNT, 2095 versus 135 for tetryl and 65 versus 8 for picric acid. The result implies that high volume ratios of IL versus aqueous stream would be preferred under economic consideration. On the contrary, in a typical set of the experiment, the phase volume ratio for TNT was changed from 0.3
3. It is because the increase in phase volume ratio increases the number of moles of ILs and enhances the interaction with TNT, tetryl and picric acid and results in higher distribution ratio. Apparently, there exists an increasing trend of distribution ratios of all the EMs with increasing volume ratios of IL versus aqueous stream. The distribution ratio of EM from aqueous stream at high volume ratios was significantly superior to that low one i.e. 1234 versus 20 for TNT, 2095 versus 135 for tetryl and 65 versus 8 for picric acid. The result implies that high volume ratios of IL versus aqueous stream would be preferred under economic consideration. On the contrary, in a typical set of the experiment, the phase volume ratio for TNT was changed from 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 0.3
3 to 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (VIL/Vaq.) and the distribution ratio was dropped from 135 to 15.
30 (VIL/Vaq.) and the distribution ratio was dropped from 135 to 15.
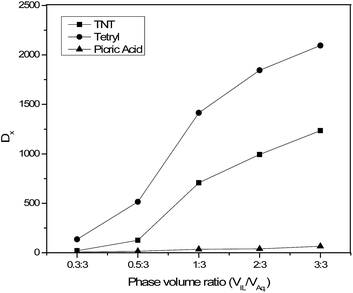 | ||
| Fig. 6 Effect of phase volume ratios of IL, [BMIM][NTf2] versus aqueous stream on distribution ratios of TNT, tetryl and picric acid (conc. 50 ppm). | ||
3.7. Repeated use of ionic liquids
The reuse of ILs is an important issue from the economic as well as environment point of view.49 For this purpose, initially, the extraction experiment of [BMIM][NTf2] with 100 ppm of TNT (pH 6.3) was performed at phase volume ratio (VIL/Vaq.) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 60 minutes of extraction time. After the extraction, the IL was separated from the aqueous phase and repeatedly treated for five times with a fresh batch of TNT (aqueous stream 3 mL, each). The extraction efficiency of IL is found to be slightly decreasing (∼1%) with an increase in the number of repeat stages for TNT extraction as shown in Fig. 7. It also indicates that the same IL can be repeated for extraction of fresh EMs up to the saturation limit of IL. The saturated IL can be recycled by washing with selected organic solvents. The recycling ability of ILs depends upon its solubility in the solvents. For instance, most of the ILs are insoluble in diethyl ether and supercritical CO2. In the present system, the ILs containing [PF6]− and [NTf2]− anion are insoluble in the organic solvents as mentioned in Table 1S.† The lack of solubility of all the selected ILs in diethyl ether allows easy stripping of the energetic material from IL phase. In a representative experiment, diethyl ether was used for the removal of TNT from IL, [BMIM][NTf2] and after few washings the IL became free from the EM. The extract and recycled IL was purified and dried in a vacuum then analyzed by IR and confirmed the absence of EM, TNT. The same IL could be reused for the further extraction process.
3 and 60 minutes of extraction time. After the extraction, the IL was separated from the aqueous phase and repeatedly treated for five times with a fresh batch of TNT (aqueous stream 3 mL, each). The extraction efficiency of IL is found to be slightly decreasing (∼1%) with an increase in the number of repeat stages for TNT extraction as shown in Fig. 7. It also indicates that the same IL can be repeated for extraction of fresh EMs up to the saturation limit of IL. The saturated IL can be recycled by washing with selected organic solvents. The recycling ability of ILs depends upon its solubility in the solvents. For instance, most of the ILs are insoluble in diethyl ether and supercritical CO2. In the present system, the ILs containing [PF6]− and [NTf2]− anion are insoluble in the organic solvents as mentioned in Table 1S.† The lack of solubility of all the selected ILs in diethyl ether allows easy stripping of the energetic material from IL phase. In a representative experiment, diethyl ether was used for the removal of TNT from IL, [BMIM][NTf2] and after few washings the IL became free from the EM. The extract and recycled IL was purified and dried in a vacuum then analyzed by IR and confirmed the absence of EM, TNT. The same IL could be reused for the further extraction process.
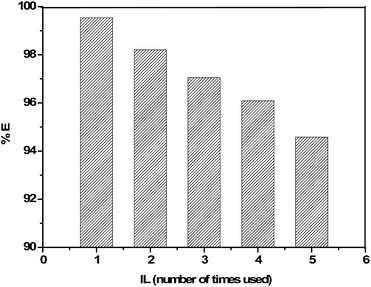 | ||
Fig. 7 Repeated use of IL, [BMIM][NTf2] for extraction of TNT from aqueous stream under the conditions of pH 6.3, VIL/Vaq. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, concentration of TNT 100 ppm and extraction time 60 min. 3, concentration of TNT 100 ppm and extraction time 60 min. | ||
4. Conclusions
An interesting conclusion stands on the point that EMs have been successfully separated from contaminated water by using IL. A role of the IL-anion is very influential in the extraction process and anion with the larger ionic radius increases the distribution ratio of EM. It is observed that higher distribution ratio of TNT occurs at acidic pH. The increase in the phase volume ratio of IL to aq. phase increases the distribution ratio, too. The extraction mechanism reveals that a C–H⋯Π interaction presents between the IL and EM. The partitioning of solutes is simple and may have potential to replace the conventional solvents. The ILs are found to be selective which may be helpful in the recovery of EMs from the composite or bipropellant. Also, the IL can be recycled by striping the EM with diethyl ether.Acknowledgements
We gratefully acknowledge the financial support from DRDO, Ministry of Defence (ERIP/ER/1003883/M/01/908/2012/D, R&D/1416, dated, 28-3-2012) New Delhi, India. One of the authors, Vikas Bhosale is thankful to the UGC, New Delhi for the Ph.D. fellowship.References
- H. Ek, Hazard Assessment of 2,4,6-trinitrotoluene (TNT) from Dumped Ammunition in the Sea, Göteborg University, 2005, p. 48 Search PubMed.
- J. D. Rodgers and N. J. Bunce, Treatment methods for the remediation of nitroaromatic explosives, Water Res., 2001, 35, 2101–2111 CrossRef CAS.
- J. Akhavan, Chemistry of Explosives, Royal Society of Chemistry, 2nd edn, 2004 Search PubMed.
- H. J. Arpe, Ullmann's Encyclopedia of Industrial Chemistry, VCH, Weinheim, 5th edn, 1991, vol. A17, p. 411 Search PubMed.
- E. P. Best, S. L. Sprecher, S. L. Larson, H. L. Fredrickson and D. F. Bader, Environmental behavior of explosives in groundwater from the Milan Army Ammunition Plant in aquatic and wetland plant treatments. Removal, mass balances and fate in groundwater of TNT and RDX, Chemosphere, 1999, 38, 3383–3396 CrossRef CAS.
- R. Alnaizy and A. Akgerman, Oxidative treatment of high explosives contaminated wastewater, Water Res., 1999, 33, 2021–2030 CrossRef CAS.
- M. E. Fuller, J. Kruczek, R. L. Schuster, P. L. Sheehan and P. M. Arienti, Bioslurry treatment for soils contaminated with very high concentrations of 2,4,6-trinitrophenylmethylnitramine (tetryl), J. Hazard. Mater., 2003, 100, 245–257 CrossRef CAS.
- S. S. Talmage, D. M. Opresko, C. J. Maxwell, C. J. Welsh, F. M. Cretella, P. H. Reno and F. B. Daniel, Nitroaromatic munition compounds: environmental effects and screening values, Rev. Environ. Contam. Toxicol., 1999, 161, 1–156 CAS.
- ATSDR, ATSDR – Toxicological Profile: 2,4,6-Trinitrotoluene (TNT), 1995.
- EPA United Sates Environmental protection Agency, 2012 Edition of the Drinking Water Standards and Health Advisories (EPA 822-S-12-001) – dwstandards2012.pdf, Washington, DC, 2012.
- C. Rajagopal and J. C. Kapoor, Development of adsorptive removal process for treatment of explosives contaminated wastewater using activated carbon, J. Hazard. Mater., 2001, 87, 73–98 CrossRef CAS.
- J. Zhang, X. Lin, X. Luo, C. Zhang and H. Zhu, A modified lignin adsorbent for the removal of 2,4,6-trinitrotoluene, Chem. Eng. J., 2011, 168, 1055–1063 CrossRef CAS PubMed.
- D. L. Freedman and K. W. Sutherland, Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) under nitrate-reducing conditions, Water Sci. Technol., 1998, 38, 33–40 CrossRef CAS.
- P. Hwang, T. Chow and N. R. Adrian, Transformation of trinitrotoluene to triaminotoluene by mixed cultures incubated under methanogenic conditions, Environ. Toxicol. Chem., 2000, 19, 836–841 CrossRef CAS.
- K. Ayoub, E. D. van Hullebusch, M. Cassir and A. Bermond, Application of advanced oxidation processes for TNT removal: A review, J. Hazard. Mater., 2010, 178, 10–28 CrossRef CAS PubMed.
- S. Chang and Y. Liu, Degradation mechanism of 2,4,6-trinitrotoluene in supercritical water oxidation, J. Environ. Sci., 2007, 19, 1430–1435 CrossRef CAS.
- D. Kalderis, S. B. Hawthorne, A. A. Clifford and E. Gidarakos, Interaction of soil, water and TNT during degradation of TNT on contaminated soil using subcritical water, J. Hazard. Mater., 2008, 159, 329–334 CrossRef CAS PubMed.
- Y. Okamoto, J. Y. Wang and E. J. Chou, Amine compound, US 4073726 A, February 14, 1978.
- Q. Zhao, Z. Ye and M. Zhang, Treatment of 2,4,6-trinitrotoluene (TNT) red water by vacuum distillation, Chemosphere, 2010, 80, 947–950 CrossRef CAS PubMed.
- Z. Meng, Q. Zhang, M. Xue, D. Wang and A. Wang, Removal of 2,4,6-Trinitrotoluene from “Pink Water” Using Molecularly-Imprinted Absorbent, Propellants, Explos., Pyrotech., 2012, 37, 100–106 CrossRef CAS.
- E. Psillakis and N. Kalogerakis, Application of solvent microextraction to the analysis of nitroaromatic explosives in water samples, J. Chromatogr. A, 2001, 907, 211–219 CrossRef CAS.
- J. G. Huddleston and R. D. Rogers, Room temperature ionic liquids as novel media for “clean” liquid–liquid extraction, Chem. Commun., 1998, 1765–1766 RSC.
- H. Zhao, S. Xia and P. Ma, Use of ionic liquids as “green” solvents for extractions, J. Chem. Technol. Biotechnol., 2005, 80, 1089–1096 CrossRef CAS.
- ed. P. Wasserscheid and T. Welton, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, http://www.wiley-vch.de/publish/en/books/ISBN978-3-527-31239-9, accessed, Sep 22, 2014.
- G.-T. Wei, Z. Yang and C.-J. Chen, Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions, Anal. Chim. Acta, 2003, 488, 183–192 CrossRef CAS.
- N. Papaiconomou, G. Vite, N. Goujon, J.-M. Lévêque and I. Billard, Efficient removal of gold complexes from water by precipitation or liquid–liquid extraction using ionic liquids, Green Chem., 2012, 14, 2050–2056 RSC.
- T. Vander Hoogerstraete, S. Wellens, K. Verachtert and K. Binnemans, Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: separations relevant to rare-earth magnet recycling, Green Chem., 2013, 15, 919–927 RSC.
- S. Génand-Pinaz, N. Papaiconomou and J.-M. Leveque, Removal of platinum from water by precipitation or liquid–liquid extraction and separation from gold using ionic liquids, Green Chem., 2013, 15, 2493–2501 RSC.
- J. McFarlane, W. B. Ridenour, H. Luo, R. D. Hunt, D. W. DePaoli and R. X. Ren, Room temperature ionic liquids for separating organics from produced water, Sep. Sci. Technol., 2005, 40, 1245–1265 CrossRef CAS PubMed.
- G. W. Meindersma, A. R. Hansmeier and A. B. de Haan, Ionic liquids for aromatics extraction. present status and future outlook, Ind. Eng. Chem. Res., 2010, 49, 7530–7540 CrossRef CAS.
- C. F. Poole and S. K. Poole, Extraction of organic compounds with room temperature ionic liquids, J. Chromatogr. A, 2010, 1217, 2268–2286 CrossRef CAS PubMed.
- J. Wang, Y. Pei, Y. Zhao and Z. Hu, Recovery of amino acids by imidazolium based ionic liquids from aqueous media, Green Chem., 2005, 7, 196–202 RSC.
- X. Li, R. Guo, X. Zhang and X. Li, Extraction of glabridin using imidazolium-based ionic liquids, Sep. Purif. Technol., 2012, 88, 146–150 CrossRef CAS PubMed.
- P. S. Kulkarni and C. A. M. Afonso, Deep desulfurization of diesel fuel using ionic liquids: current status and future challenges, Green Chem., 2010, 12, 1139–1149 RSC.
- A. Seeberger and A. Jess, Desulfurization of diesel oil by selective oxidation and extraction of sulfur compounds by ionic liquids—a contribution to a competitive process design, Green Chem., 2010, 12, 602–608 RSC.
- C. Asumana, G. Yu, X. Li, J. Zhao, G. Liu and X. Chen, Extractive desulfurization of fuel oils with low-viscosity dicyanamide-based ionic liquids, Green Chem., 2010, 12, 2030–2037 RSC.
- C. Yansheng, L. Changping, J. Qingzhu, L. Qingshan, Y. Peifang, L. Xiumei and U. Welz-Biermann, Desulfurization by oxidation combined with extraction using acidic room-temperature ionic liquids, Green Chem., 2011, 13, 1224–1229 RSC.
- P. S. Kulkarni and V. K. Bhosale, Extraction and recovery of energetic materials using ionic liquids, Patent 2731/MUM/2013, 2013.
- P. S. Kulkarni, L. C. Branco, J. G. Crespo, M. C. Nunes, A. Raymundo and C. A. M. Afonso, Comparison of physicochemical properties of new ionic liquids based on imidazolium, quaternary ammonium and guanidinium cations, Chem.–Eur. J., 2007, 13, 8478–8488 CrossRef CAS PubMed.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation, Green Chem., 2001, 3, 156–164 RSC.
- C. F. Poole, Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids, J. Chromatogr. A, 2004, 1037, 49–82 CrossRef CAS PubMed.
- U.S. EPA Method 8330, Nitroaromatics, nitramines and nitrate esters by high performance liquid chromatography (HPLC), 2006.
- C. C. Cassol, A. P. Umpierre, G. Ebeling, B. Ferrera, S. S. X. Chiaro and J. Dupont, On the extraction of aromatic compounds from hydrocarbons by imidazolium ionic liquids, Int. J. Mol. Sci., 2007, 8, 593–605 CrossRef CAS PubMed.
- R. Bini, O. Bortolini, C. Chiappe, D. Pieraccini and T. Siciliano, Development of cation/anion “interaction” scales for ionic liquids through ESI-MS measurements, J. Phys. Chem. B, 2007, 111, 598–604 CrossRef CAS PubMed.
- M. G. Freire, C. M. S. S. Neves, P. J. Carvalho, R. L. Gardas, A. M. Fernandes, I. M. Marrucho, L. M. N. B. F. Santos and J. A. P. Coutinho, Mutual solubilities of water and hydrophobic ionic liquids, J. Phys. Chem. B, 2007, 111, 13082–13089 CrossRef CAS PubMed.
- R. P. Swatloski, J. D. Holbrey and R. D. Rogers, Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate, Green Chem., 2003, 5, 361–363 RSC.
- J. C. Lynch, K. F. Myers, J. M. Brannon and J. J. Delfino, Effects of pH and temperature on the aqueous solubility and dissolution rate of 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), J. Chem. Eng. Data, 2001, 46, 1549–1555 CrossRef CAS.
- W.-S. Chen, W.-C. Chiang and K.-M. Wei, Recovery of nitrotoluenes from wastewater by solvent extraction enhanced with salting-out effect, J. Hazard. Mater., 2007, 147, 197–204 CrossRef CAS PubMed.
- B. Wu, W. Liu, Y. Zhang and H. Wang, Do we understand the recyclability of ionic liquids?, Chem.–Eur. J., 2009, 15, 1804–1810 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR and physico-chemical properties of ionic liquids, and HPLC chromatogram of representative samples. See DOI: 10.1039/c4ra17271j |
| This journal is © The Royal Society of Chemistry 2015 |