Synthesis of α-Fe2−xAgxO3 nanocrystals and study of their optical, magnetic and antibacterial properties†
Mayank Bhushana,
S. Muthukamalamb,
S. Sudharanib and
Annamraju Kasi Viswanath*a
aCentre for Nanoscience and Technology, Pondicherry University, Puducherry-605014, India. E-mail: v_kasi@hotmail.com
bDepartment of Biochemistry and Molecular Biology, Pondicherry University, Puducherry-605014, India
First published on 25th March 2015
Abstract
To be an implicit disinfectant, inorganic nanoparticles have to show chemical stability, minimum cytotoxicity and effective bactericidal activity. Among metal oxide nanoparticles, iron oxide demonstrates a very high structural stability in corrosive biological environments and also has a relatively non-toxic profile in comparison with other metal nanoparticles such as ZnO. Iron oxide nanoparticles also exhibit bacterial growth inhibition properties on a wide spectrum of bacterial species mainly by generating reactive oxygen species (ROS) from water and oxygen. However, the efficiency of their antibacterial activity remains low. Probably, the main reason behind this is the aggregation and occurrence of large flocculates of nanoparticles in aqueous media due to their hydrophobic nature and hence their interaction with bacteria is limited. To overcome all these problems, in this study we incorporate silver ions into α-Fe2O3 to produce magnetic hybrid nanostructures with better colloidal stability and enhanced antibacterial activity due to their synergistic effect. The antibacterial activity of the prepared nanospheres was tested at 3 different concentrations (450, 600, 750 μg) against four bacterial strains – Bacillus subtilis, Staphylococcus aureus, Pseudomonas fluorescens and Escherichia coli using a disc diffusion method. The nanospheres showed a concentration dependent activity profile and remarkably, they were very effective against B. subtilis and P. fluorescens. Their antibacterial effect was found to be comparable to the standard antibiotic streptomycin used in this study. Furthermore, in this work structural, optical and magnetic properties of the prepared samples have been studied using different characterization tools.
1. Introduction
On a large scale, the evolution of nanotechnology started with the semiconductor industry due to a continuous demand for low dimensional materials with better electrical and optical properties for device fabrication.1–5 Because of their extremely small size range, nanomaterials exhibit a wide variation in their physical, chemical and physiological properties compared to their bulk counterparts. Here lies the opportunity to tune the properties of nanoparticles based on our requirements. Antibacterial materials play an important role in maintaining human health by providing efficient treatment for the infectious diseases caused by pathogenic bacteria.6,7 Considering the wide range of antibacterial agents available today, silver based nanomaterials have received much attention due to their high throughput and wide spectrum bactericidal activity.8–10 These inorganic nanostructures have distinct advantages over conventional chemical bactericides as they suffer through multidrug resistance. In general, the bactericidal property of chemical agents relies on their specific binding with microbial cell wall or, surface and metabolism of agents into the micro-organism. A range of pathogenic micro-organisms have evolved drug resistance during course of time over many generations. So far, these chemical based conventional antimicrobial agents have been effective for therapy; however, their use has been confined to medical instruments and in prophylaxis in antimicrobial facilities. Hence, to find an unorthodox way to overcome the drug resistance of wide range of pathogenic microorganisms is very much needed on urgent basis. Silver ions and silver salts have been known for their growth-inhibitory property against microorganisms and have been used for the same in past. Also, activity of other metal ions like Cu,11,12 Zn13,14 and Mg15 against microorganisms have been reported by various research groups. However the usefulness of Ag ions or salts as such as an antimicrobial agent has got some limitations due to various reasons, including the restraining effects of salts. In sharp contrast, these kinds of shortcomings can be overcome by the use of Ag nanoparticles which produce enhanced antimicrobial property in comparison with the bulk. The mechanism behind this high bactericidal activity is that silver cations, i.e., Ag+ ions could damage the bacteria by binding with the thiol groups of proteins and also by interrupting with DNA replication mechanism which leads to loss of DNA replication ability of bacteria and hence eventually causes death of bacteria.16,17 In addition, antibacterial behavior of silver has also been reported specifically due to inactivation of enzyme phosphomannose isomerase.18 Therefore high throughput of antibacterial activity of silver based compounds mainly depends on controlled and durable generation of Ag+ ions. There are few limitations which restrict the use of silver salt and silver nanoparticles, constituents of a number of commercial bactericidal products, in their further application in many areas.19 The main reason behind this is abrupt release of Ag+ ions from the silver salt, causing potential danger for human life and environmental safety. The applications of Ag nanoparticles are also limited by occurrence of large aggregations and murky Ag+ ions release, resulting into loss of antibacterial activity.20 To solve the issues mentioned above, integration or, innovation of nanostructured silver based nanomaterials with prolonged and efficient bactericidal activity is the need of hour. So, to use bactericidal property of Ag in various fields against pathogenic and drug resistant microorganisms, it is essentially required to prepare the Ag based systems with cost-effective methods and also to know the mechanism involved in causing the antimicrobial effect. Also, it is very much crucial to intensify the antimicrobial effect. Antibacterial properties of α-Fe2O3 nanoparticles and their composites on different bacteria have been reported by few groups21–23 and also antibacterial studies of Ag@Fe3O4 and γ-Fe2O3@Ag has been reported by R. Prucek et al.24 Recently, antibacterial properties of iron oxide @ carbon nanocomposites have been reported against E. coli and S. aureus.25,26 In this study we report the cost-effective preparation of Ag doped α-Fe2O3 homogeneous and stable nanoparticles. The nanosize of the hybrid system has provided enhanced surface area with the microorganisms, and also due to its nanoscale size it can easily be applied to medical devices using surface coating methods.Study of magnetic properties of nanostructures has drawn significant scientific surveillance due to their multi-disciplinary prospective applications such as in biotechnology,27–30 electronics, magnetic fluids, catalysis, memory devices31 and environmental treatment. Recently, various groups have worked on detection of bacterial pathogens (Pseudomonas aeruginosa)32 and viral pathogens like hepatitis B33,34 and porcine endogenous retrovirus (PERV)35 based on magnetic properties of iron oxide nanoparticles and chemiluminescence. Also, these properties have been used to develop an analytical system to analyze copy number variation of DNA.36 To accomplish these applications, different approaches have been used for preparing magnetic nanocomposites with many distinct compositions.37–40 As iron oxide nanoparticles exhibit minimum level of cytotoxicity compared with other metal oxide nanoparticles,41 along with drug carrier and MRI contrast agent they have also been used for detecting overexpression of cyclooxygenase-2 (COX-2) and B-cell lymphoma-2 (BCL-2) associated with gastric cancer.42,43 Iron oxide (α-Fe2O3) nanomaterials exhibit a classic rhombohedral crystal structure and are preferred while preparing magnetic hybrid nanocomposites because of their inherent magnetic properties associated with the nanoscale size and surface effects. The FexOy nanomaterials are generally susceptible to high magnetic response in presence of an externally enforced magnetic field and hence possess a potential use in recyclable nanocatalysis, selective capturing of intended substrates and magneto-photonic purposes.44–50 Here, in this work, we report the synthesis of α-Fe2−xAgxO3 systems for the first time which is Ag doped hematite nanostructures through chemical co-precipitation method and study of their structural, optical, magnetic and antibacterial properties against four different bacterial species (both gram positive and gram negative).
2. Materials and methods
2.1. Materials
Ferrous sulphate (FeSO4·7H2O), silver nitrate (AgNO3), sodium hydroxide (NaOH) and Sodium Dodecyl Sulphate (SDS) were purchased from Sigma Aldrich, India. Ethanol, nutrient broth and nutrient agar media were purchased from Hi-media, India.2.2. Preparation of nanoparticles
Ferrous sulphate heptahydrate (0.1 M) and respective weight of AgNO3 corresponding to 1, 3, 5 and 7 atomic weight% of ferrous sulphate heptahydrate were dissolved in 100 ml of double distilled water in four different beakers (250 ml) and allowed for proper mixing for 2 h by stirring using a magnetic stirrer. After that 1% wt/vol of SDS was added to all the beakers and allowed for stirring for 1 h followed by addition of 50 ml of NaOH solution (0.4 M) drop wise in all the beakers under continuous stirring. All the solutions were kept for overnight stirring and then washed thrice with double distilled water and once with ethanol using ultracentrifugation operated at 800 rpm for 10 minutes each time. The resulting samples were dried in hot air oven at 32 °C for 1 h and used for further characterization after annealing at 800 °C for 3 h. One more sample was prepared by adopting same procedure but without adding AgNO3. Details and name of all the samples is given in Table 1.| S. N. | Sample code | Composition | Atomic wt% of Ag | %wt/vol of SDS used for synthesis |
|---|---|---|---|---|
| 1 | IS | α-Fe2O3 | 0 | 1 |
| 2 | ISS1 | α-Fe1.99Ag0.01O3 | 1 | 1 |
| 3 | ISS2 | α-Fe1.97Ag0.03O3 | 3 | 1 |
| 4 | ISS3 | α-Fe1.95Ag0.05O3 | 5 | 1 |
| 5 | ISS4 | α-Fe1.93Ag0.07O3 | 7 | 1 |
2.3. Characterization
The prepared samples were used to study the structural events using Rigaku Ultima IV X-ray Diffractometer with Cu Kα = 1.5418 Å, operating voltage 40 kV, current 40 mA and step size 0.02° in 2θ scan. SEM micrographs were recorded by Hitachi, Model S-3400N equipped with energy dispersive spectroscope. The Raman spectra were obtained with Confocal-Raman spectrometer excited by Ar+ laser 488 nm lines. The Thermo NicoletModel6700 Fourier transform infrared spectroscopy (FTIR) was used to confirm the chemical bonds and the nature of the sample. Perkin Elmer λ650 UV-Visible spectrophotometer was used for UV-Visible absorbance spectrum. Thermal behavior of the sample was studied by Model Q600 SDT and Q20 DSC Thermo Gravimetric analysis (TG/DTA) and the magnetic properties were characterized by vibrating sample magnetometer VSM Lake Shore 7404 at room temperature. All the characterizations were done according to standard operational procedures.2.4. Antibacterial activity of α-Fe2−xAgxO3 nanospheres
3. Results and discussion
3.1. XRD
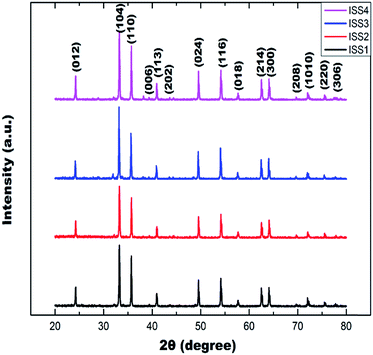
XRD patterns of Ag doped and undoped nanosized α-Fe2O3 samples were obtained after calcining the as prepared products at 800 °C for 3 h and can be seen in Fig. 1. All the sharp and strong diffraction peaks present in Fig. 1 can be assigned to α-Fe2O3 with a hexagonal structure which is well consistent with the values in literature (JCPDS No: 33-0664). There is no extra peak analogous to Ag, oxides of Ag related secondary and impurity phases evident from the XRD spectra, which may be referred to the embodiment of Ag ions into the Fe lattice site rather than interstitial ones. The average crystallite size of nanoparticles was calculated using Debye - Scherrer's52 equation:
D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
| Sample code | Avg. crystallite size (nm) | 2θ | Planes |
|---|---|---|---|
| IS | 37.13 | 24.24 | (012) |
| 33.27 | (104) | ||
| 35.74 | (110) | ||
| ISS1 | 39.79 | 40.93 | (113) |
| 49.55 | (024) | ||
| ISS2 | 41.51 | 54.17 | (116) |
| 57.69 | (018) | ||
| ISS3 | 44.08 | 62.52 | (214) |
| 64.07 | (300) | ||
| ISS4 | 41.87 | 69.64 | (208) |
| 72.03 | (1010) | ||
| 75.51 | (220) | ||
| 77.78 | (306) |
3.2. UV-Vis spectra
The optical band gaps of all annealed samples were measured using UV-Vis absorption spectroscopic technique. The absorbance curves for all annealed samples are shown in Fig. 2 and it is clearly observed that the absorption maxima shifted from 534 nm to 570 nm as the silver content increases in the sample and also a slight decrease in intensity of absorption peaks has been observed with the increased silver content in the sample.There are three explicit absorption regions present in the UV-Vis absorption spectra of the undoped and Ag doped α-Fe2O3 nanoparticles. As per the references,53,54 absorption between 250–400 nm primarily results from the ligand to metal charge-transfer transitions and slightly from the contribution of the Fe3+ ligand field transitions 6A1 → 4T1 (4P) at 290–310 nm, 6A1 → 4E (4D) and 6A1 → 4T2 (4D) at 360–380 nm. Absorption in the region between 400–600 nm is considered to arise due to the pair excitation processes 6A1 + 6A1 → 4T1 (4G) + 4T1 (4G) at 485–550 nm, probably imbricated the contributions of 6A1 → 4E, 4A1 (4G) ligand field transitions at 430 nm and the charge-transfer band tail. Absorption in wavelength region between 600–800 nm is related to the 6A1 → 4T2 (4G) transition at about 640 nm. However, the absorption intensity in regions between 250–400 nm and 400–600 nm is much larger than that in region between 600–800 nm, which implies that the absorption due to the charge-transfer transitions or the pair excitations is much stronger than the absorption due to the ligand field transitions because of the selection rules.
The optical band gap, Eg (for a direct transition between the valence and conduction band), was obtained from diffuse reflectance spectra by plotting the square of the product of Kubelka–Munk function F(R) and energy as a function of energy. Kubelka–Munk function F(R) is estimated by using the relation
| F(R) = (1 − R)2/2R |
3.3. Raman spectrum
The Raman spectra of undoped and Ag doped α-Fe2O3 samples annealed at 800 °C for 3 h were taken and are shown in Fig. 4. The α-Fe2O3 with corundum-type crystal structure is the most common iron oxide on earth and seven phonon lines are expected to appear in the Raman spectrum, namely two A1g modes (223 and 496 cm−1) and five Eg modes (256, 292, 299, 408 and 607 cm−1).50 Our samples exhibit all the peaks corresponding to the typical frequencies observed for α-Fe2O3, as seen in the Raman spectra. However, some shifts were observed which may occur due to differences in size and shape of the particles. The sharp peak at around 1314 cm−1 is due to a two-magnon scattering which emerges from the correlation of two magnons created on antiparallel close spin sites.55 The characteristic peaks at 666, 820, 1050 and 1103 cm−1 are observed only in highly crystallined hematite,56 as our samples exhibit these peaks which again confirms the high crystalline nature of the prepared samples.3.4. Thermal analysis
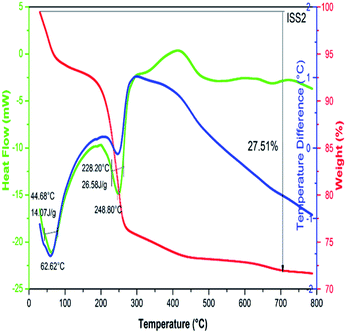
Thermogravimetric (TGA) analysis and Differential Scanning Calorimetry (DSC) were executed for undoped and Ag doped α-Fe2O3 nanoparticles, to know the phase formation and decomposition which occurs in the course of heat treatment. The thermal analysis was carried out from room temperature to 800 °C. TGA of as prepared samples was performed by heating in air atmosphere at 5°C min−1 in an alumina crucible. The DSC–TGA curve for the sample ISS2 is shown in Fig. 5 and TGA curves for all the samples are given in Fig. 2 of ESI.† It is observed that the mass loss appears in a wide range of temperature from room temperature up to 660 °C. The specific mass loss at temperatures below 100 °C illustrates the existence of free water or physiosorbed water. Further, the extended downfall of relative mass versus temperature implies that the surface water molecules possibly remain in an immense set of energetically nonequivalent surface hydration groups. When the temperature is over to 640 °C the sample mass eventually becomes constant. From the mass difference in TGA data, the total water content was determined to be as high as 27.51 wt%. The downward peak in heat flow graph and further decrease of mass was observed at 248.8 °C and 420.2 °C in weight curve, which represent decompositions of Fe(OH)2 and Ag(OH)2 respectively. Both the materials liberate water molecules. The two exothermic peaks noticed in the DTA curve around 500 and 600 °C are because of the burning of impurities. Beyond 640 °C, a flatter weight loss curve is seen because of the release of water originating from the condensation reaction. These results were in good agreement with the literature.573.5. VSM analysis
To study the magnetic behavior and the evolution of magnetism in pure and Ag doped α-Fe2O3 samples, measurements were made using a Vibrating Sample magnetometer at room temperature. Fig. 6 shows the magnetic hysteresis loops of the annealed samples at various doping levels. α-Fe2O3 nanoparticles often exhibit the unusual magnetic behavior.58 Presence of the free surface and grain boundaries leads to the improved magnetic properties.59 The results demonstrate that they have weak ferromagnetic behavior. The saturation magnetization was not observed in the applied range of magnetization field. Table 3 shows the values of coercivity (Hc) and remanent magnetization (Mr). For samples ISS2 and ISS3, the values were almost approximate. However, the samples ISS1 and IS exhibited a higher value for Mr and shows strong hysteresis loop behavior. The ISS1 sample displayed the highest magnetic properties with Mr of 0.1672 emu g−1 and Hc of 1933 Oe. It must be due to the influence of Ag ion doping in hematite lattice. The variation of the magnetism with the increase of Ag-doping suggests that the crystallization quality of Fe2−xAgxO3 and the formation of the second phase also have great effect on the regular arrangement of the magnetic moment. Since the increase in Ag concentration is helpful to enhance the exchange interaction of the Ag ions between different bound magnetic polarons (BMP) areas, the ferromagnetism first increases with the increase of Ag-doping and then decreases. Therefore, the magnetization also has a doping dependent behavior for α-Fe2O3 samples.| Sample code | Coercivity (Gauss) | Retentivity (emu g−1) × 10−6 | Magnetization (emu g−1) × 10−3 |
|---|---|---|---|
| IS | 1366.66 | 0.1344 | 0.4177 |
| ISS1 | 1933.33 | 0.1672 | 0.4832 |
| ISS2 | 2355.55 | 0.1298 | 0.3606 |
| ISS4 | 2355.55 | 0.1403 | 0.3744 |
3.6. Scanning electron micrograph (SEM) and EDAX
The size and morphology of all the annealed α-Fe2O3 samples were examined by SEM analysis. Fig. 7(A) shows the SEM image of sample ISS2, and it demonstrates that the nanoparticles have got agglomerated to some extent and possess almost spherical or, oval shape. Fig. 7(B) shows the EDS spot spectra of sample ISS2 (α-Fe1.97Ag0.03O3), showing the surface elemental components: iron, oxygen and silver.3.7. Analysis of antibacterial activity
The prepared undoped and doped Fe2O3 nanospheres are expected to exhibit antibacterial activity because of their robust Ag+ ions release ability and also because of inherent native bactericidal property of hematite nanocrystals giving a synergistic effect in enhancing the bactericidal properties of hematite nanocrystals. The antibacterial activity of the samples ISS1, ISS2, ISS3, ISS4 and IS was assessed against four bacterial strains i.e., E. coli, B. subtilis, P. fluorescens and S. aureus by the disk diffusion method and the inhibition zones produced by them are shown in Fig. 8(A–D). Figure for zone inhibition of sample IS against tested bacteria (8E) is given in ESI.†The principle of the Bauer–Kirby disc diffusion susceptibility test is to determine the sensitivity of pathogenic aerobic/facultative bacteria to various antimicrobial compounds. The paper disks impregnated with known concentrations of test compound when placed on agar plate, water is absorbed from the agar into the disks and the antimicrobial compound begins to diffuse into the surrounding agar. The presence or absence of growth around the disk is an indirect measure of the ability of that compound to inhibit that organism.
The results obtained in this study clearly indicated that diameter of inhibition zone depends upon the concentration of Fe2O3 nanospheres and as the concentration of nanospheres increases from 450 to 750 μg the inhibition zone diameter also increases. In our experiment, we have analyzed the effect of Ag doping in α-Fe2O3 nanospheres on the inhibition zone diameter by taking 450 μg, 600 μg and 750 μg from each of the IS, ISS1, ISS2, ISS3 and ISS4 samples respectively and got clear, significant and increasing inhibition zone with increasing concentration of the samples as well as with increasing Ag content in the samples, suggesting an effective antibacterial effect. The maximum inhibition zone was observed at concentrations of 600 & 750 μg. The zone of inhibition produced by ISS3 & ISS4 against all the four bacterial strains used in this study was found to be equivalent and even improved as compared to the positive control streptomycin. The diameters of inhibition zone (in mm) for different concentrations of all the samples are tabulated in Table 4.
| Concn of nano particles (in μg) | Zone of inhibition (in mm) | |||||
|---|---|---|---|---|---|---|
| IS | ISS1 | ISS2 | ISS3 | ISS4 | Streptomycin | |
| Bacillus subtilis | ||||||
| 450 | 10 | 10 | 12 | 16 | 19 | 20 |
| 600 | 10 | 12 | 13 | 17 | 20 | |
| 750 | 10 | 13 | 16 | 19 | 20 | |
| Pseudomonas fluorescens | ||||||
| 450 | 10 | 10 | 10 | 17 | 17 | 19 |
| 600 | 10 | 11 | 12 | 19 | 20 | |
| 750 | 10 | 12 | 13 | 20 | 20 | |
| Staphylococcus aureus | ||||||
| 450 | 9 | 10 | 10 | 10 | 10 | 15 |
| 600 | 9 | 10 | 12 | 14 | 16 | |
| 750 | 10 | 11 | 12 | 16 | 16 | |
| Escherichia coli | ||||||
| 450 | 9 | 10 | 12 | 13 | 14 | 18 |
| 600 | 10 | 10 | 13 | 16 | 19 | |
| 750 | 10 | 12 | 13 | 19 | 20 | |
Furthermore, to establish the antibacterial effect of the nanomaterials, their effect on bacterial growth pattern against the E. coli, B. subtilis, P. fluorescens and S. aureus was monitored by measuring the optical density (OD) at 600 nm based on the turbidity of the cell suspension. The bacterial cultures were treated with five different concentrations 45 mg dl−1, 60 mg dl−1, 75 mg dl−1, 90 mg dl−1 and 120 mg dl−1 of the samples ISS1, ISS2, ISS3, ISS4 and IS respectively and the OD values were recorded for 24 h at regular intervals of time and a graph was plotted between optical density and time as shown in Fig. 9(A–D). Graph between optical density and time for sample IS (Fig. 9E) is given in ESI. The control flasks without treatment showed robust bacterial growth. On the other hand, reduced bacterial growth was observed in the presence of Fe2−xAgxO3 nanocrystals even at a concentration as low as 45 mg dl−1. Interestingly, treatment with ISS3 & ISS4 at the concentration of 120 mg dl−1 resulted in complete growth inhibition in all the four bacterial strains used in this study (Fig. 9C and D). Similar effect was brought about by ISS1 and ISS2 with P. fluorescens (Fig. 9A and B). This observation clearly indicates that, as the concentration of undoped and doped Fe2O3 nanospheres increases the optical density decreased, measured bacterial growth was diminished whereas the control sample shows the growth rate increased with the time. From this analysis it was found that Fe2O3 nanospheres at 120 mg dl−1 concentration, show effective antibacterial property leading to no or very less bacterial growth. The minimum inhibitory concentration (MIC) varied with the samples. In the case of samples IS and ISS1 minimum inhibitory concentration was found to be 90 mg dl−1 against all the strains. The MIC value for sample ISS2 was found to be 75 mg dl−1 and for samples ISS3 and ISS4 the MIC value was 60 mg dl−1 against all the strains.
4. Conclusions
We have successfully fabricated nanostructures of Ag doped α-Fe2O3 by using simple, reliable and cost effective chemical route method. The Fe2−xAgxO3 nanostructures were characterized by using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), UV-Visible spectroscopy, Raman spectroscopy and vibrating sample magnetometer. The antibacterial activity of Fe2−xAgxO3 nanostructures were evaluated using inhibition zone and growth curve analysis and are found to be an effective and durable antimicrobial agents, showing enhanced antimicrobial activity due to synergistic effect of Ag+ ion and native hematite nanostructures.Acknowledgements
This research was supported by the UGC university research fellowship, Govt. of India. Authors express their thanks to Central Instrumentation Facility Head of Pondicherry University Dr G. Govindrajan for allowing to characterize our samples through various instruments available at the centre and we are also thankful to the CIF's staff members for their help and co-operation during characterization process.References
- A. K. Viswanath, Encyclopedia of Nanoscience and Nanotechnology, American Scientific Publishers, Los Angeles, California, USA, 2004 Search PubMed.
- A. K. Viswanath, Handbook of Semiconductor Nanostructures and Nanodevices, American Scientific Publishers, Los Angeles, California, USA, 2006 Search PubMed.
- A. K. Viswanath, Handbook of Surfaces and Interfaces of Materials, Academic Press, 2001 Search PubMed.
- A. K. Viswanath, J. Nanosci. Nanotechnol., 2014, 14, 1253 CrossRef CAS PubMed.
- A. K. Viswanath, J. Nanosci. Nanotechnol., 2014, 14, 1947 CrossRef CAS PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301 CrossRef CAS PubMed.
- K. Fukushima, S. Liu, H. Wu, A. C. Engler, D. J. Coady, H. Maune, J. Pitera, A. Nelson, N. Wiradharma, S. Venkataraman, Y. Huang, W. Fan, J. Y. Ying, Y. Y. Yang and J. L. Hedrick, Nat. commun., 2013, 4, 2861 Search PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76 CrossRef CAS PubMed.
- A. Panacek, L. Kvitek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. Nevecna and R. Zboril, J. Phys. Chem. B, 2006, 110, 16248 CrossRef CAS PubMed.
- J. R. Nakkala, R. Mata, A. K. Gupta and S. R. Sadras, Eur. J. Med. Chem., 2014, 85, 784 CrossRef CAS PubMed.
- B. Bussa, P. S. Rao, S. Muthukamalam, S. Ramachitra, S. Sudha Rani and T. Swu, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2013, 43, 1073 CrossRef CAS.
- A. K. Chatterjee, R. Chakraborty and T. Basu, Nanotechnology, 2014, 25, 135101 CrossRef PubMed.
- R. K. Dutta, B. P. Nenavathu, M. K. Gangishetty and A. V. R. Reddy, Colloids Surf., B, 2012, 94, 143 CrossRef CAS PubMed.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, Appl. Environ. Microbiol., 2011, 77, 2325 CrossRef CAS PubMed.
- Z. X. Tang, X. J. Fang, Z. L. Zhang, T. Zhou, X. Y. Zhang and L. E. Shi, Braz. J. Chem. Eng., 2012, 29, 775 CrossRef CAS PubMed.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662 CrossRef CAS.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346 CrossRef CAS PubMed.
- T. N. C. Wells, P. Scully, A. E. Proudfoot and M. A. Payton, Biochemistry, 1995, 20, 7896 CrossRef.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 145, 83 CrossRef CAS PubMed.
- X. Li and J. J. Lenhart, Environ. Sci. Technol., 2012, 46, 5378 CrossRef CAS PubMed.
- J. Singh, M. Srivastava, J. Dutta and P. K. Duttaa, Int. J. Biol. Macromol., 2011, 48, 170 CrossRef CAS PubMed.
- P. Basnet, G. K. Larsen, R. P. Jadeja, Y. C. Hung and Y. Zhao, ACS Appl. Mater. Interfaces, 2013, 5, 2085 CAS.
- M. M. Rafi, K. S. Z. Ahmed, K. P. Nazeer, D. S. Kumar and M. Thamilselvan, Appl. Nanosci., 2015, 5, 515 CrossRef CAS.
- R. Prucek, J. Tucek, M. Kilianova, A. Panacek, L. Kvitek, J. Filip, M. Kolar, K. Tomankova and R. Zboril, Biomaterials, 2011, 32, 4704 CrossRef CAS PubMed.
- S. F. Situ and A. C. S. Samia, ACS Appl. Mater. Interfaces, 2014, 6, 20154 CAS.
- T. Tian, X. Shi, L. Cheng, Y. Luo, Z. Dong, H. Gong, L. Xu, Z. Zhong, R. Peng and Z. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 8542 CAS.
- S. H. Hu, C. H. Tsai, C. F. Liao, D. M. Liu and S. Y. Chen, Langmuir, 2008, 24, 11811 CrossRef CAS PubMed.
- X. Mou, Z. Ali, S. Li and N. He, J. Nanosci. Nanotechnol., 2015, 15, 54 CrossRef CAS PubMed.
- A. Ito, M. Shinkai, H. Honda and T. Kobayashi, J. Biosci. Bioeng., 2005, 100, 1 CrossRef CAS PubMed.
- S. G. Grancharov, H. Zeng, S. Sun, S. X. Wang, S. O'Brien, C. B. Murray, J. R. Kirtley and G. A. Held, J. Phys. Chem. B, 2005, 109, 13030 CrossRef CAS PubMed.
- S. H. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS.
- Y. Tang, Z. Ali, J. Zou, K. Yang, X. Mou, Z. Li, Y. Deng, Z. Lu, C. Ma, M. A. A. Shah, S. Elingarami, H. Yang and N. He, J. Nanosci. Nanotechnol., 2014, 14, 4886 CrossRef CAS PubMed.
- H. Yang, W. Liang, N. He, Y. Deng and Z. Li, ACS Appl. Mater. Interfaces, 2015, 7, 774 CAS.
- F. Wang, C. Ma, X. Zeng, C. Li, Y. Deng and N. He, J. Biomed. Nanotechnol., 2012, 8, 786 CrossRef CAS PubMed.
- H. Yang, Z. Li, Q. Jiang, J. Fan, B. Zhou, Y. Guo, G. Lan, X. Yang, N. He and H. Jiang, J. Nanosci. Nanotechnol., 2015, 15, 5597 CrossRef PubMed.
- M. Liu, P. Hu, G. Zhang, Y. Zeng, H. Yang, J. Fan, L. Jin, H. Liu, Y. Deng, S. Li, X. Zeng, S. Elingarami and N. He, Theranostics, 2015, 4, 71 CrossRef PubMed.
- J. Kim, J. E. Lee, J. Lee, Y. Jang, S. W. Kim, K. An, J. H. Yu and T. Hyeon, Angew. Chem., Int. Ed., 2006, 45, 4789 CrossRef CAS PubMed.
- S. Sacanna and A. P. Philipse, Langmuir, 2006, 22, 10209 CrossRef CAS PubMed.
- M. Chen, Y. N. Kim, H. M. Lee, C. Li and S. O. Cho, J. Phys. Chem. C, 2008, 112, 8870 CAS.
- D. Nagao, I. Yokoyama, N. Yamauchi, H. Matsumoto, Y. Kobayashi and M. Konno, Langmuir, 2008, 24, 9804 CrossRef CAS PubMed.
- S. Maiti, J. Biomed. Nanotechnol., 2011, 7, 65 CrossRef CAS PubMed.
- Y. Zhang, H. Liu, Y. Jia, Y. Deng, S. Li and N. He, Sci. Adv. Mater., 2015, 7, 532 CrossRef PubMed.
- Y. Zhang, Y. Jia, S. Li, Y. Deng, H. Liu and N. He, Sci. Adv. Mater., 2014, 6, 1146 CrossRef CAS PubMed.
- D. K. Yi, S. S. Lee and J. Y. Ying, Chem. Mater., 2006, 18, 2459 CrossRef CAS.
- G. Lee, J. Kim and J. H. Lee, Enzyme Microb. Technol., 2008, 42, 466 CrossRef CAS PubMed.
- J. Ge, H. Lee, L. He, J. Kim, Z. Lu, H. Kim, J. Goebl, S. Kwon and Y. Yin, J. Am. Chem. Soc., 2009, 131, 15687 CrossRef CAS PubMed.
- J. Ge, L. He, Y. Hu and Y. Yin, Nanoscale, 2011, 3, 177 RSC.
- H. H. Yang, S. Q. Zhang, X. L. Chen, Z. X. Zhuang, J. G. Xu and X. R. Wang, Anal. Chem., 2004, 76, 1316 CrossRef CAS PubMed.
- H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. Sun, Nano Lett., 2005, 5, 379 CrossRef CAS PubMed.
- D. Tang, R. Yuan and Y. Chai, J. Phys. Chem. B, 2006, 110, 11640 CrossRef CAS PubMed.
- A. W. Bauer, W. M. M. Kirby, J. C. Sherris and M. Turck, Am. J. Clin. Pathol., 1966, 45, 493 CAS.
- H. P. Klug, and L. E. Alexander, X-Ray Diffraction Procedure, Wiley InterScience, New York, 1954, pp. 504–524 Search PubMed.
- Y. P. He, Y. M. Miao, C. R. Li, S. Q. Wang, L. Cao, S. S. Xie, G. Z. Yang and B. S. Zou, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 125411 CrossRef.
- D. M. Sherman and T. D. Waite, Am. Mineral., 1985, 70, 1262 CAS.
- L. Chen, X. Yang, J. Chen, J. Liu, H. Wu, H. Zhan, C. Liang and M. Wu, Inorg. Chem., 2010, 49, 8411 CrossRef CAS PubMed.
- D. Bersani, P. P. Lottici and A. Montenero, J. Raman Spectrosc., 1999, 30, 355 CrossRef CAS.
- B. Ahmmad, K. Leonard, M. S. Islam, J. Kurawaki, M. Muruganandham, T. Ohkubo and Y. Kuroda, Adv. Powder Technol., 2013, 24, 160 CrossRef CAS PubMed.
- H. Ni, Y. Ni, Y. Zhou and J. Hong, Mater. Lett., 2012, 73, 206 CrossRef CAS PubMed.
- B. B. Straumal, A. A. Mazilkin, S. G. Protasova, A. A. Myatiev, P. B. Straumal and E. Goering, Thin Solid Films, 2011, 520, 1192 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra17259k |
| This journal is © The Royal Society of Chemistry 2015 |